30 March 2024: Clinical Research
Potential Value of HSP90α in Prognosis of Triple-Negative Breast Cancer
Han Fei Wang12CDEF, Ying Chen12G, Bang Cao3E, Jing Pei12A*DOI: 10.12659/MSM.943049
Med Sci Monit 2024; 30:e943049
Abstract
BACKGROUND: Triple-negative breast cancer (TNBC) is a distinct subtype of breast cancer, accounting for 12-18% of all breast cancer cases. It exhibits high heterogeneity and aggressiveness, resulting in a poorer prognosis with a high risk of early recurrence and metastasis. Due to the lack of expression of estrogen receptors (ER), progesterone receptors (PR), and human epidermal growth factor receptor 2 (HER2), as well as insensitivity to endocrine therapy, determining a standard treatment for TNBC is challenging. The identification of potential prognostic biomarkers is crucial for developing personalized treatment strategies for patients.
MATERIAL AND METHODS: Our study investigated the potential value of HSP90a in TNBC prognosis. A retrospective analysis was conducted on 127 TNBC patients and 127 Healthy controls from March 1, 2019 to July 31, 2022. Venous blood was collected and tested for HSP90α, CEA, CA199, and CA125, and we recorded the clinical characteristics of the patients, including age, BMI, alcohol consumption status, surgical history, CEA level, CA199 level, CA125 level, HSP90α level, tumor size, distant metastases, lymph node metastasis, and TNM stage. Univariate and multivariate methods were used to screen independent risk factors for progression-free survival (PFS) and overall survival (OS).
RESULTS: HSP90α is not only upregulated in TNBC but is also highly correlated with lymph node metastasis and TNM stage. The results of multivariate analysis showed that distant metastasis, TNM stage and HSP90a level were independent factors associated with PFS. BMI, tumor size, TNM stage, surgical history, and HSP90a level were independent factors influencing OS.
CONCLUSIONS: Our research findings demonstrate a significant association between high HSP90α expression and adverse clinical features, suggesting a poorer prognosis for TNBC patients.
Keywords: Prognosis, HSP90AA1 Protein, Human, Triple Negative Breast Neoplasms
Introduction
Breast cancer is the most often diagnosed cancer worldwide and is among the most common cancers among women. It also contributes significantly to mortality from cancer [1]. Breast cancer is becoming the most common cancer among Chinese women, as it is in many other nations; cases in China account for 12.2% of all newly diagnosed breast cancers and 9.6% of all deaths from breast cancer worldwide [2].
Triple-negative breast cancer (TNBC) is an aggressive subtype, accounting for 12–18% of all breast cancer cases. Patients with TNBC lack estrogen receptors (ER), progesterone receptors (PR), and HER2 receptors, making them unsuitable for hormone or HER2-targeted therapies. Compared to other subtypes, TNBC is biologically and clinically more aggressive, with a poorer prognosis and a higher risk of early recurrence [3,4]. Marra et al found that TNBC patients have higher T and N staging and lower histological differentiation compared to other breast cancer subtypes [5].
Because of TNBC’s genetic variability and a lack of targetable molecular modifications, post-treatment survival results show only minimal improvement, and there are no long-term viable therapy alternatives [4]. Currently, neoadjuvant chemotherapy (NAC) is the conventional treatment for many TNBC patients, which consists of a combination of taxane-based medications (mitotic inhibitors) and anthracycline-based therapies (DNA intercalators). While NAC is beneficial for certain TNBC patients, around 50% develop medication resistance, resulting in a low overall survival rate [6,7]. TNBC is the subtype with the worst prognosis due to tumor heterogeneity and a long-standing dearth of effective therapeutics beyond chemotherapy [8].
Furthermore, the incidence of metastasis is increasing, with estimates ranging from 20% to 30% of breast cancer patients experiencing metastasis after diagnosis and original tumor treatment. Metastasis is responsible for approximately 90% of cancer-related fatalities, with non-metastatic breast cancer patients having a 5-year overall survival rate of more than 80%, while distant metastasis reduces this rate to 25% [9–11]. The typical time to recurrence for non-TNBC patients is 35–67 months, whereas it is just 19–40 months for TNBC patients, with a 75% mortality rate within 3 months after recurrence [12,13].
Because of the insensitivity to molecular targeting and endocrine therapies, the distinct molecular phenotype, poor response to conventional postoperative adjuvant chemotherapy, and the scarcity of molecular biology research on TNBC, reliable prognostic markers for TNBC patients have not been developed. As a result, there is an urgent need to identify a sensitive and specific TNBC tumor marker that can enhance patient prognosis and survival and lay the groundwork for future TNBC research.
HSP (heat shock protein) chaperones are a type of molecular chaperone. Cells increase the expression of HSPs in response to environmental stresses such as heat, heavy metals, hypoxia, and acidosis [14]. The HSP family is split into HSP27, HSP40, HSP60, HSP70, HSP90, and big HSP based on molecular weight. Members of this family are linked together by signaling networks [15]. The HSP90 family includes many ATP-dependent molecular chaperones that aid in the folding of newly generated proteins or the stabilization of denatured proteins in response to stress [16,17]. Chircop et al discovered that the cellular stress response is important in cancer progression and treatment [7,18]. HSP90 regulates and protects several malignant activities, including tumor development, adhesion, invasion, metastasis, and angiogenesis. HSP90 is highly expressed in a variety of malignant tumors, indicating that cancer cells are under heightened pressure to preserve protein integrity and, as a result, a higher need for HSP90 [19]. Furthermore, HSP90 overexpression in tumor tissues is frequently associated with a poor prognosis [20]. Inhibiting HSP90 can cause oncoprotein degradation and disruption of oncogenic pathways [21].
Many oncoproteins have mutant versions, and their stability and activity are strongly reliant on HSP90 molecular chaperones [22]. In model systems utilizing fruit flies and Arabidopsis, Whitesell et al revealed that HSP90 can operate as a biochemical buffer, giving cancer cells tolerance to mutant proteins and the potential to modify signaling pathways [23]. Zhang et al discovered that angiogenesis is inhibited in breast cancer by blocking the VEGF-related pathway with HSP90 inhibitors [24]. Kim et al discovered that HSP90 interacts with the hTERT (human telomerase reverse transcriptase) promoter in immortalized tumor cells, and that blocking HSP90 can lead to decreased hTERT expression [25]. Research has provided preliminary evidence of the role of HSP90 in the development of cancer and the therapeutic potential of HSP90 inhibitors. HSP90α, a protein product of HSP90AA1, is thought to play an important role in tumor invasion and migration [26]. Liu et al examined the diagnostic value of plasma HSP90α in breast cancer by using an online database and clinical parameters. They selected 146 patients with benign breast disease and 566 patients with stage I, II, and III breast cancer as the cancer risk cohort. Among breast cancer patients, they included 566 stage I–III patients and 109 stage IV patients as the metastasis risk cohort. Nomograms models were constructed for assessing the risk of breast cancer and breast cancer metastasis based on logistic regression analysis. Seven indicators – HSP90α, CA153, CEA, NK, monocyte count, B cells, and D-dimer – were incorporated into both models. The study revealed that HSP90α levels combined with other markers could effectively predict patients’ cancer and metastasis rates [27].
There is an urgent need to explore the role of HSP90α in cancer development and its potential as a therapeutic target, and there is currently a lack of research regarding plasma HSP90α in TNBC. The objective of this study was to explore the potential of plasma HSP90α as a diagnostic and prognostic marker in TNBC. A retrospective analysis was carried out on the clinical data of 127 patients with TNBC, and the inclusion criteria, the parameters evaluated, and the detection procedure of the plasma HSP90α are provided. The results suggested a significant correlation between plasma HSP90α levels, clinical characteristics, and survival of TNBC patients. This article demonstrated that plasma HSP90α was overexpressed in patients with TNBC and was linked to adverse clinicopathologic features, revealing that significantly high HSP90α levels in patients with TNBC were closely associated with OS and PFS.
Material and Methods
GENERAL INFORMATION:
A retrospective analysis was performed on clinical data from March 1, 2019, to July 31, 2022, covering 127 patients diagnosed with triple-negative breast cancer (TNBC) who were admitted to Anhui Medical University’s First Affiliated Hospital. There were also 127 healthy controls included, for a total of 254 female participants. The participants’ ages ranged from 28 to 86 years, with the TNBC group having a median age of 62 years. Chemotherapy, targeted therapy, immunotherapy, and combination therapies were administered to the TNBC group. The healthy control group’s median age was 61 years.
ETHICS STATEMENT:
Approval was obtained from the First Affiliated Hospital of Anhui Medical University Committee on Medical Ethics (approval no. PJ2023-13-60). The requirement for patient informed consent was waived because of the retrospective nature of the study.
INCLUSION CRITERIA:
We included patients with a diagnosis of TNBC confirmed by pathology, and who had not received systemic treatment, such as chemotherapy or immunotherapy, or biologic treatments, without malignant tumors, and without systemic inflammatory disorders. The healthy controls were without no malignant tumors or systemic inflammatory disorders.
METHODS:
Age, body mass index (BMI), alcohol consumption history, surgical history, CEA (carcinoembryonic antigen) level, CA199 (carbohydrate antigen199) level, CA125 (carbohydrate antigen125) level, HSP90α level, tumor size, distant metastases, lymph node metastasis, and TNM stage were all examined. We used the TNM stage approach established by the International Union Against Cancer and the American Joint Committee on Cancer. The study assessed progression-free survival (PFS) and overall survival (OS). PFS refers to the time from the start of randomization to the first recorded disease progression or death. OS is defined as the time from the start of randomization to death due to any cause.
PLASMA HSP90α DETECTION:
All participants provided 4-mL fasting venous blood samples collected in EDTA-K2 anticoagulant tubes. Plasma HSP90α levels were detected using ELISA reagents (Protgen Ltd, Yantai, China). The cutoff value for HSP90α was set at 81.09, and the area under the curve (AUC) was 0.72. Based on the cutoff value, the 127 TNBC patients were categorized into a low-value and high-value groups, comprising 75 and 52 individuals, respectively (Figure 1, Table 1). The 4-mL venous blood samples were centrifuged at 3000 rpm for 10 min to separate serum for the detection of tumor markers. CEA (carcinoembryonic antigen), CA199, and CA125 were assessed using an automated chemiluminescence analyzer and corresponding reagent kits, and the positive criteria for these tumor markers were CEA ≥5 ng/ml, CA199 ≥40 U/ml, and CA125 ≥35 U/ml, respectively.
STATISTICAL ANALYSIS:
Age was not normally distributed and thus is expressed as median. Enumeration data are expressed as counts or percentages, and HSP90α levels differences were assessed by
Results
PLASMA HSP90α LEVELS IN PATIENTS:
The plasma HSP90α level in the TNBC group was 107.94±23.20 ng/ml, while it was 67.16±33.15 ng/ml in the Healthy Control group. The plasma HSP90α level in the TNBC group was significantly higher than in the Healthy Control group (P value=0.0020) (Table 2, Figure 2). The rate of high HSP90α level (≥81.09 ng/ml) in TNBC patients was 40.62%, while it was 13.39% in the Healthy Control group. The rate of high HSP90α level was much greater in the TNBC group than in the Healthy Control group, with a statistically significant difference (P=0.001) (Table 2).
RELATIONSHIP BETWEEN PLASMA HSP90α LEVELS AND CLINICAL CHARACTERISTICS:
The plasma HSP90α levels in the TNBC group and the Healthy Control group were significantly different with respect to lymph node metastasis (P<0.05) and TNM stage (P<0.001) (Table 3).
CORRELATION BETWEEN HSP90α LEVELS AND SURVIVAL:
Results from univariate survival analysis revealed that patients with normal HSP90α levels (2.6 months), M0 stage (3.0 months), and TNM stage 1+2 (9.6 months), and those who underwent surgery (4.2 months) and had T stage 1+2 (2.5 months), and normal CA199 levels (2.7 months) had longer PFS compared to patients with abnormal HSP90α levels (2.6 months), M1 stage (1.1 months), and TNM stage 3+4 (1.2 months), and those who did not undergo surgery (1.2 months), had T stage 3+4 (1.2 months), and abnormal CA199 levels (1.2 months) (Figure 3).
Patients with normal HSP90α levels (25.8 months), low BMI (24.0 months), M0 stage (32.2 months), TNM stage 1+2 (undefined), those who underwent surgery (undefined), T stage 1+2 (45.0 months), and had normal CA199 levels (29.4 months) had longer OS compared to patients with abnormal HSP90α levels (19.3 months), high BMI (12.6 months), M1 stage (11.7 months), TNM stage 3+4 (12.6 months), and those who did not undergo surgery (12.6 months), had T stage 3+4 (12.4 months), and abnormal CA199 levels (12.6 months) (Figure 4).
Multivariate analysis was conducted on the variables that showed statistically significant differences in univariate analysis. The results of the multivariate analysis indicated that distant metastasis, TNM stage, and HSP90α levels were factors independently influencing PFS. BMI, tumor size, TNM stage, whether surgery was performed, and HSP90α levels were independent influencing factors for OS (Table 4).
Discussion
Plasma HSP90αhas been shown to have diagnostic and prognostic utility in various malignancies, but its role in TNBC remains unknown. The clinical importance of plasma HSP90α in TNBC patients was investigated in this study, which discovered that HSP90α levels in TNBC patients were considerably higher than those in healthy controls. We found that plasma HSP90α levels was associated with lymph node metastasis and TNM stage in TNBC patients, implying a close relationship between plasma HSP90α and TNBC etiology. Furthermore, we discovered that plasma HSP90α showed prognostic value.
Breast cancer is a diverse set of diseases with varying morphology, genomic features, and clinical behavior. TNBC is a major cause of cancer-related fatalities, and TNBC diagnosis and treatment strategies rely heavily on early imaging and laboratory investigations. There is now substantial dispute over the best therapy regimens.
Due to the lack of target proteins such as estrogen receptors, cytotoxic chemotherapy has been the mainstay of treatment for decades. Immunotherapy has emerged as a first-line treatment for various malignancies. Tumors can inhibit the immune response by activating negative regulatory pathways associated with immune homeostasis. The interaction between tumor cells and the host immune system promotes immune escape by tumors, leading to tumor spread, recurrence, and metastasis. Immune checkpoint inhibitors can disrupt immune inhibitory signals, maintain immune homeostasis, and enhance the body’s anti-tumor immunity. Immunotherapy has altered the treatment landscape and prognosis of aggressive malignancies. TNBC patients, compared to other breast cancer subtypes, have higher immunogenicity, making them derive the greatest benefit from immunotherapy [28–31]. However, this heterogeneity also presents challenges in understanding the biology and mechanisms of breast cancer progression. Further insights into tumor biology have facilitated the identification of subgroups of patients with specific molecular characteristics and advanced the exploration of this disease [32].
Hsp90 is well acknowledged as a critical molecular chaperone and master regulator in important cellular signaling networks in human cells [33]. Hsp90 has been shown in vitro and in vivo to accelerate tumor development, induce epithelial-mesenchymal transition (EMT), increase cell motility, and contribute to treatment resistance [34,35]. Hsp90 expression is controlled by the cell cycle in normal cells and is continually expressed at high levels in tumor cells even in the absence of heat stress [36]. This evidence emphasizes the importance of Hsp90 in cancer development and progression. The majority of HSP90α in gastric cancer tissues is found in the cytoplasm, with only a small percentage identified in the nucleus [37]. Moreover, breast cancer tissues exhibit higher levels of HSP90α expression when compared to normal non-cancerous tissues [38]. Our study, focusing on its role in TNBC, revealed significantly elevated levels of plasma HSP90α in the TNBC group compared to the Healthy Control group, with statistically significant differences. This lays a foundation for further uncovering the tumor biology mechanisms of TNBC. A study showed that overexpression of HSP90α in breast cancer is associated with shorter OS and aggressive clinicopathological features, including advanced clinical stage, large tumors, and lymph node metastasis [39].
TNBC, the most aggressive subtype of breast cancer, has a significant rate of recurrence and mortality in operable stages (I–III), resulting in a shorter overall survival period [40]. This work confirms HSP90α’s relationship with TNBC, notably its substantial link with lymph node and distant metastasis, highlighting its potential as a useful discriminator of TNBC metastasis. High HSP90α expression is related with negative clinical and pathological features of TNBC, and our study demonstrates that distant metastasis, TNM stage, and HSP90α levels are independent factors affecting patients’ PFS.
BMI, tumor size, TNM stage, surgical status, and HSP90αlevels have been established as independent predictors of OS. This study is the first to show a link between survival in TNBC patients and high HSP90α expression, demonstrating that high HSP90α expression is related with shorter survival, which is similar with the findings of Fayaz et al [41]. The 10-year overall survival rates for TNBC patients were reported at 66%, correlating with disease stage (stage 1–92%, stage 2–80%, stage 3–49%, stage 4-0). Notably, the absence of recurrence, distant metastasis, and local recurrence accounted for 59%, 72%, and 77%, respectively.
Previous research has revealed that elevated HSP90α expression is an independent predictor of TNBC mortality [42]. Liu and colleagues discovered a link between HSP90α and overall survival, with patients with high HSP90α expression having worse overall survival [43]. High HSP90α expression levels are related with poor overall survival, as validated by biostatisticians who analyzed HSP90α expression in nearly 4000 breast cancer patients from 23 databases [42].
Conclusions
In this single-center retrospective study, we found that plasma HSP90α is overexpressed in TNBC patients and is linked to common tumor indicators. High expression of HSP90α was significantly associated with poor prognostic features such as lymph node metastasis and higher TNM stage, which provides important information for patient outcomes. The results of the analysis further showed that distant metastasis, TNM stage, and HSP90α levels are independent factors affecting patients’ PFS. Also, BMI, tumor size, TNM stage, surgical status, and HSP90α levels have been established as independent predictors of OS. In summary, HSP90α is a parameter that can be obtained from a simple and inexpensive test as a useful marker in predicting long-term survival in TNBC patients, having a very strong predictive ability and utility. The conclusions of this study need to be confirmed by studies with larger sample sizes or further randomized controlled trials.
Figures
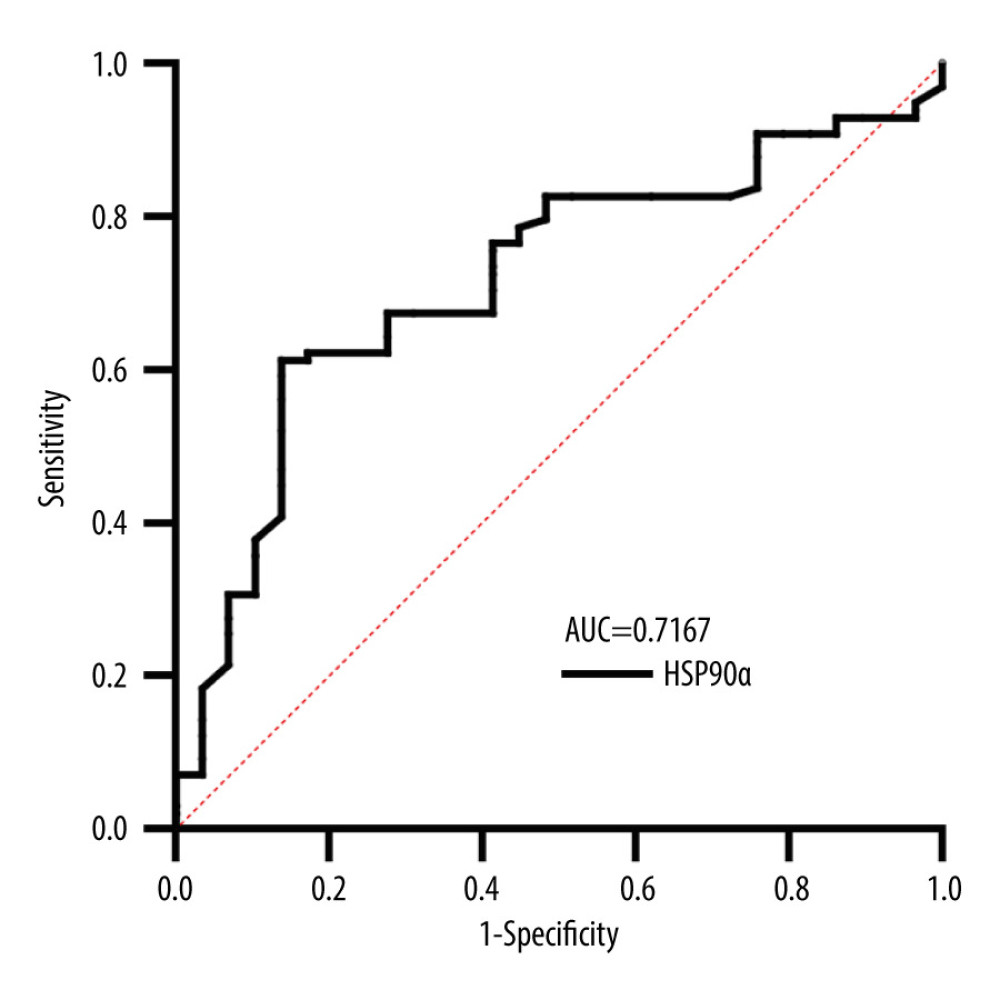 Figure 1. Receiver operating characteristic (ROC) curve for HSP90α in Predicting Overall Survival (OS). Produced using GraphPad Prism 9.5 (GraphPad, La Jolla, California, USA).
Figure 1. Receiver operating characteristic (ROC) curve for HSP90α in Predicting Overall Survival (OS). Produced using GraphPad Prism 9.5 (GraphPad, La Jolla, California, USA). 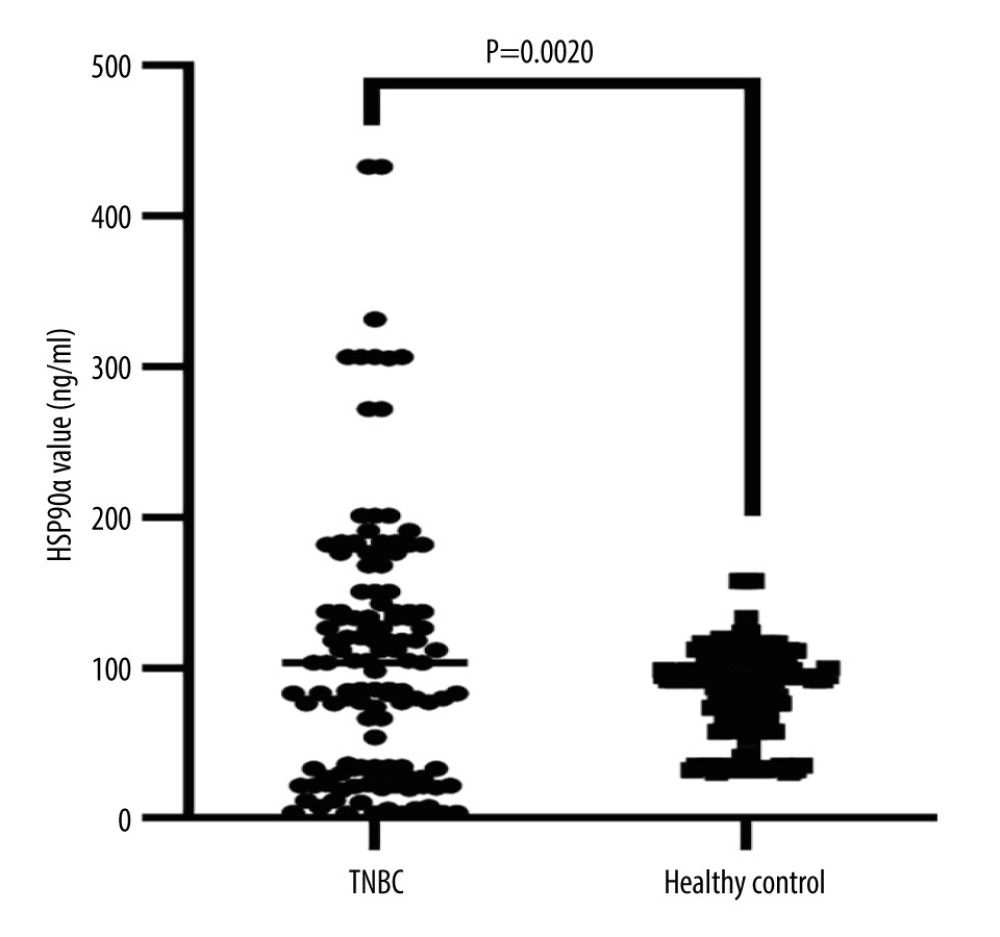 Figure 2. HSP90α value in health controls and patients with TNBC. Produced using GraphPad Prism 9.5 (GraphPad, La Jolla, California, USA).
Figure 2. HSP90α value in health controls and patients with TNBC. Produced using GraphPad Prism 9.5 (GraphPad, La Jolla, California, USA). 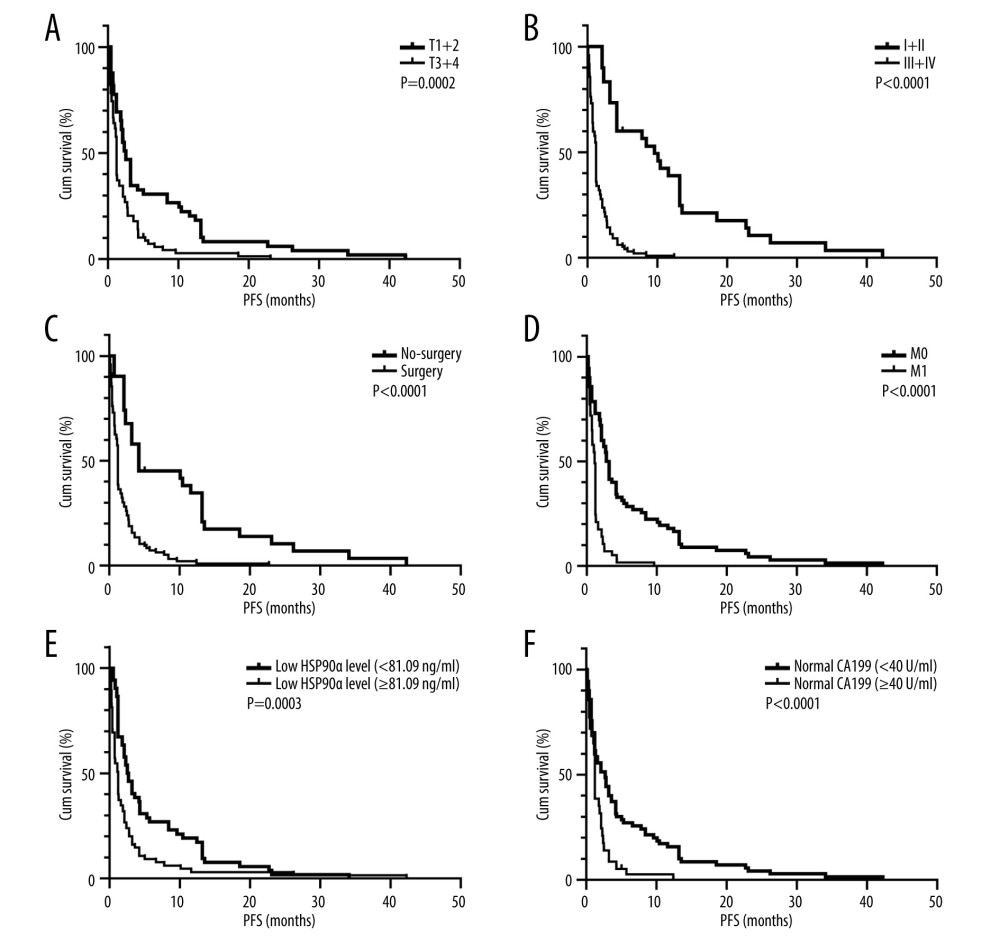 Figure 3. (A–F) K-M curves of different variables for PFS in 127 patients with TNBC. Produced using GraphPad Prism 9.5 (GraphPad, La Jolla, California, USA).
Figure 3. (A–F) K-M curves of different variables for PFS in 127 patients with TNBC. Produced using GraphPad Prism 9.5 (GraphPad, La Jolla, California, USA). 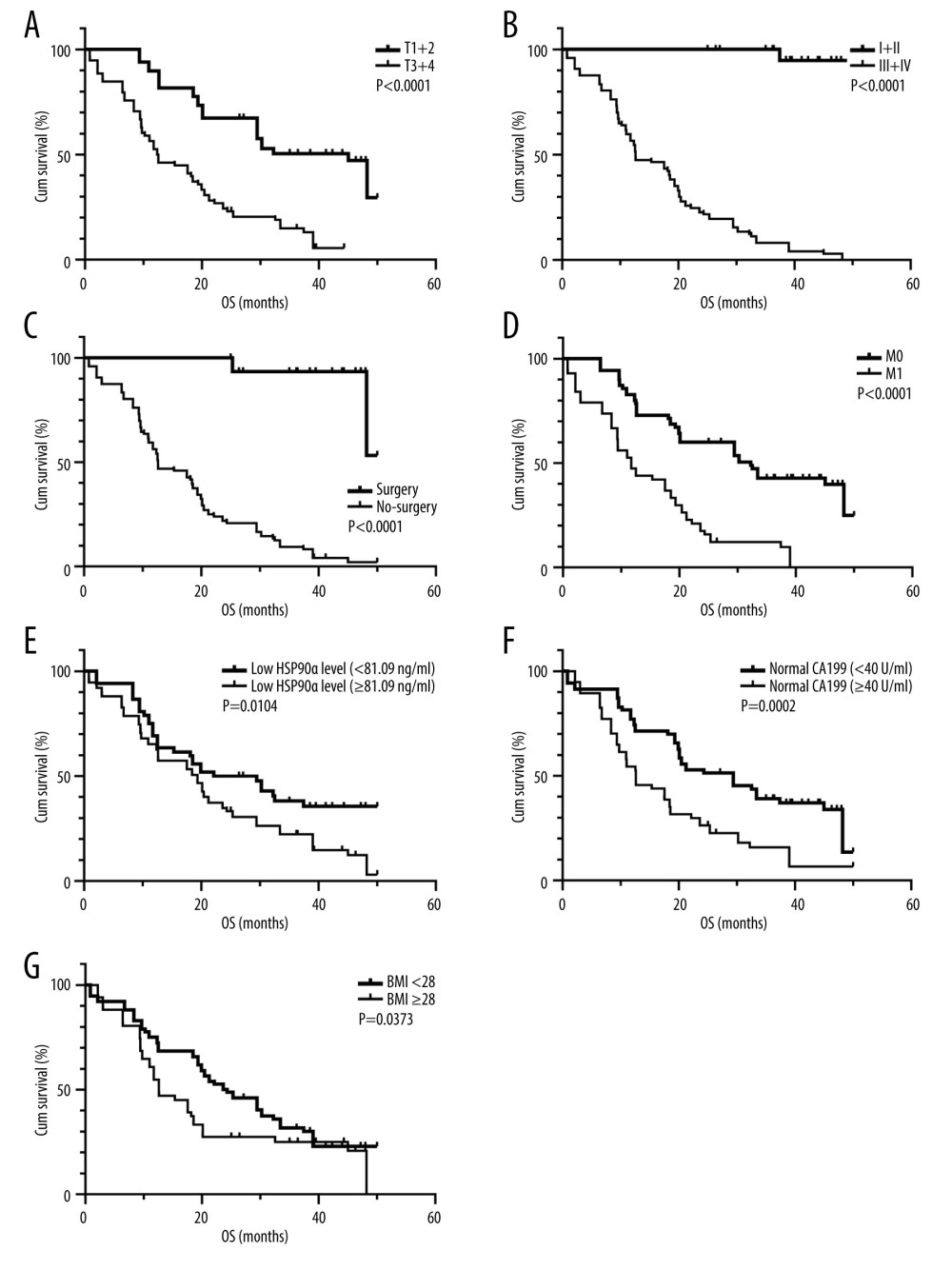 Figure 4. (A–G) K-M curves of different variables for OS in 127 patients with TNBC. Produced using GraphPad Prism 9.5 (GraphPad, La Jolla, California, USA).
Figure 4. (A–G) K-M curves of different variables for OS in 127 patients with TNBC. Produced using GraphPad Prism 9.5 (GraphPad, La Jolla, California, USA). References
1. Allemani C, Matsuda T, Di Carlo V, Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries: Lancet, 2018; 391(10125); 1023-75
2. Fan L, Strasser-Weippl K, Li JJ, Breast cancer in China: Lancet Oncol, 2014; 15(7); e279-89
3. Karim AM, Eun Kwon J, Ali T, Triple-negative breast cancer: Epidemiology, molecular mechanisms, and modern vaccine-based treatment strategies: Biochem Pharmacol, 2023; 212; 115545
4. Foulkes WD, Smith IE, Reis-Filho JS, Triple-negative breast cancer: N Engl J Med, 2010; 363(20); 1938-48
5. Marra A, Trapani D, Viale G, Practical classification of triple-negative breast cancer: Intratumoral heterogeneity, mechanisms of drug resistance, and novel therapies: NPJ Breast Cancer, 2020; 6(1); 54
6. Liedtke C, Mazouni C, Hess KR, Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer: J Clin Oncol, 2008; 26(8); 1275-81
7. Bianchini G, De Angelis C, Licata L, Treatment landscape of triple-negative breast cancer-expanded options, evolving needs: Nat Rev Clin Oncol, 2022; 19(2); 91-113
8. Valastyan S, Weinberg R, Tumor metastasis: Molecular insights and evolving paradigms: Cell, 2011; 147(2); 275-92
9. Navin NE, Hicks J, Tracing the tumor lineage: Mol Oncol, 2010; 4(3); 267-83
10. Kessenbrock K, Plaks V, Werb Z, Matrix metalloproteinases: Regulators of the tumor microenvironment: Cell, 2010; 141(1); 52-67
11. Zhang L, Fang C, Xu X, Androgen receptor, EGFR, and BRCA1 as biomarkers in triple-negative breast cancer: A meta-analysis: Biomed Res Int, 2015; 2015; 357485
12. Gluz O, Liedtke C, Gottschalk N, Triple-negative breast cancer – current status and future directions: Ann Oncol, 2009; 20(12); 1913-27
13. Whitesell L, Lindquist SL, HSP90 and the chaperoning of cancer: Nat Rev Cancer, 2005; 5(10); 761-72
14. Wu J, Liu T, Rios Z, Heat shock proteins and cancer: Trends Pharmacol Sci, 2017; 38(3); 226-56
15. Butler LM, Ferraldeschi R, Armstrong HK, Maximizing the therapeutic potential of HSP90 inhibitors: Mol Cancer Res, 2015; 13(11); 1445-51
16. Scaltriti M, Dawood S, Cortes J, Molecular pathways: Targeting hsp90 – who benefits and who does not: Clin Cancer Res, 2012; 18(17); 4508-13
17. Chircop M, Speidel D, Cellular stress responses in cancer and cancer therapy: Front Oncol, 2014; 4; 304
18. Isaacs JS, Xu W, Neckers L, Heat shock protein 90 as a molecular target for cancer therapeutics: Cancer Cell, 2003; 3(3); 213-17
19. Žáčková M, Moučková D, Lopotová T, Hsp90 – a potential prognostic marker in Cml: Blood Cells Mol Dis, 2013; 50(3); 184-89
20. Patel K, Wen J, Magliocca K, Heat shock protein 90 (Hsp90) is overexpressed in P16-negative oropharyngeal squamous cell carcinoma, and its inhibition in vitro potentiates the effects of chemoradiation: Cancer Chemother Pharmacol, 2014; 74(5); 1015-22
21. Jhaveri K, Ochiana SO, Dunphy MP, Heat shock protein 90 inhibitors in the treatment of cancer: Current status and future directions: Expert Opin Investig Drugs, 2014; 23(5); 611-28
22. Ciocca DR, Calderwood SK, Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications: Cell Stress Chaperones, 2005; 10(2); 86-103
23. Whitesell L, Lindquist SL, HSP90 and the chaperoning of cancer: Nat Rev Cancer, 2005; 5(10); 761-72
24. Zhang P-C, Liu X, Li M-M, AT-533, a novel Hsp90 inhibitor, inhibits breast cancer growth and HIF-1α/VEGF/VEGFR-2-mediated angiogenesis in vitro and in vivo. Biochem: Pharmacol, 2020; 172; 113771
25. Wong DS, Jay DG, Emerging roles of extracellular Hsp90 in cancer: Adv Cancer Res, 2016; 129; 141-63
26. Kim RH, Kim R, Chen W, Association of hsp90 to the hTERT promoter is necessary for hTERT expression in human oral cancer cells: Carcinogenesis, 2008; 29(12); 2425-31
27. Liu H, Zhang Z, Huang Y, Plasma HSP90AA1 predicts the risk of breast cancer onset and distant metastasis: Front Cell Dev Biol, 2021; 9; 639596
28. Schmid P, Cortes J, Pusztai L, Pembrolizumab for early triple-negative breast cancer: N Engl J Med, 2020; 382(9); 810-21
29. Cortes J, Cescon DW, Rugo HS, Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial: Lancet, 2020; 396(10265); 1817-28
30. Mittendorf EA, Zhang H, Barrios CH, Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial: Lancet, 2020; 396(10257); 1090-100
31. Nanda R, Liu MC, Yau C, Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with early-stage breast cancer: An analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial: JAMA Oncol, 2020; 6(5); 676-84
32. Dawson SJ, Rueda OM, Aparicio S, A new genome-driven integrated classification of breast cancer and its implications: EMBO J, 2013; 32(5); 617-28
33. Ding WJ, Ji YY, Jiang YJ, Gephyromycin C, a novel small-molecule inhibitor of heat shock protein Hsp90, induces G2/M cell cycle arrest and apoptosis in PC3 cells in vitro: Biochem Biophys Res Commun, 2020; 531(3); 377-82
34. Hance MW, Dole K, Secreted Hsp90 is a novel regulator of the epithelial to mesenchymal transition (EMT) in prostate cancer: J Biol Chem, 2012; 287(45); 37732-44
35. Whitesell L, Santagata S, Mendillo ML, HSP90 empowers evolution of resistance to hormonal therapy in human breast cancer models: Proc Natl Acad Sci USA, 2014; 111(51); 18297-302
36. Liu XL, Xiao B, Yu ZC, Down-regulation of Hsp90 could change cell cycle distribution and increase drug sensitivity of tumor cells. World J: Gastroenterol, 1999; 5(3); 199-208
37. Zuo DS, Dai J, Bo AH, Significance of expression of heat shock protein90alpha in human gastric cancer: World J Gastroenterol, 2003; 9(11); 2616-18
38. Lin T, Qiu Y, Peng W, Heat shock protein 90 family isoforms as prognostic biomarkers and their correlations with immune infiltration in breast cancer: Biomed Res Int, 2020; 2020; 2148253
39. Klimczak M, Biecek P, Zylicz A, Heat shock proteins create a signature to predict the clinical outcome in breast cancer: Sci Rep, 2019; 9(1); 7507
40. Lin NU, Vanderplas A, Hughes ME, Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network: Cancer, 2012; 118(22); 5463-72
41. Fayaz S, Demian GA, El-Sherify M, Triple negative breast cancer: 10-year survival update of the applied treatment strategy in Kuwait: Gulf J Oncolog, 2019; 1(29); 53-59
42. Cheng Q, Chang JT, Geradts J, Amplification and high-level expression of heat shock protein 90 marks aggressive phenotypes of human epidermal growth factor receptor 2 negative breast cancer: Breast Cancer Res, 2012; 14(2); R62
43. Liu H, Zhang Z, Huang Y, Plasma HSP90AA1 predicts the risk of breast cancer onset and distant metastasis: Front Cell Dev Biol, 2021; 9; 639596
Figures
 Figure 1. Receiver operating characteristic (ROC) curve for HSP90α in Predicting Overall Survival (OS). Produced using GraphPad Prism 9.5 (GraphPad, La Jolla, California, USA).
Figure 1. Receiver operating characteristic (ROC) curve for HSP90α in Predicting Overall Survival (OS). Produced using GraphPad Prism 9.5 (GraphPad, La Jolla, California, USA). Figure 2. HSP90α value in health controls and patients with TNBC. Produced using GraphPad Prism 9.5 (GraphPad, La Jolla, California, USA).
Figure 2. HSP90α value in health controls and patients with TNBC. Produced using GraphPad Prism 9.5 (GraphPad, La Jolla, California, USA). Figure 3. (A–F) K-M curves of different variables for PFS in 127 patients with TNBC. Produced using GraphPad Prism 9.5 (GraphPad, La Jolla, California, USA).
Figure 3. (A–F) K-M curves of different variables for PFS in 127 patients with TNBC. Produced using GraphPad Prism 9.5 (GraphPad, La Jolla, California, USA). Figure 4. (A–G) K-M curves of different variables for OS in 127 patients with TNBC. Produced using GraphPad Prism 9.5 (GraphPad, La Jolla, California, USA).
Figure 4. (A–G) K-M curves of different variables for OS in 127 patients with TNBC. Produced using GraphPad Prism 9.5 (GraphPad, La Jolla, California, USA). Tables
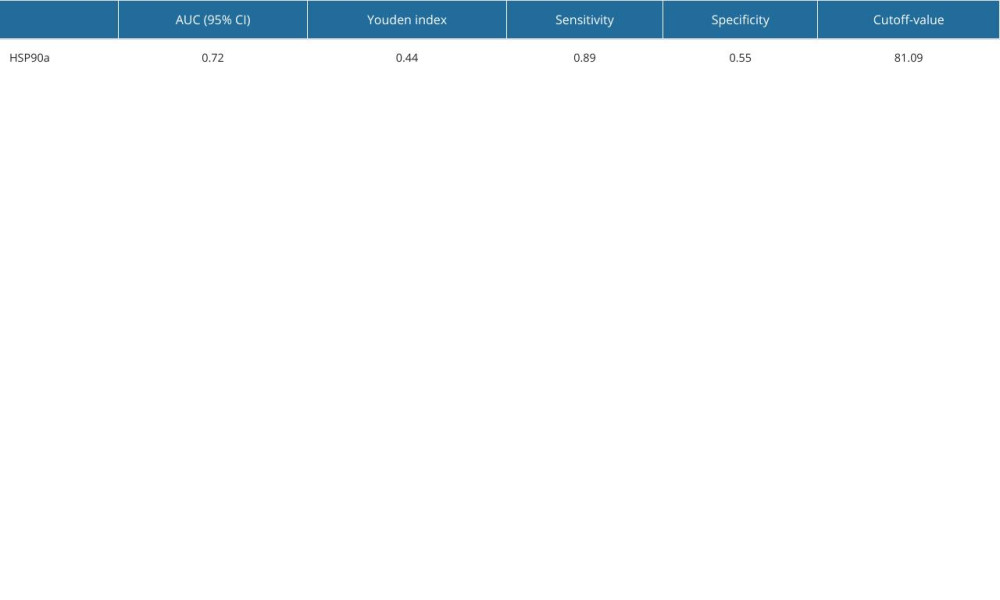 Table 1. Cutoff value and OS diagnostic efficacy of HSP90α.
Table 1. Cutoff value and OS diagnostic efficacy of HSP90α.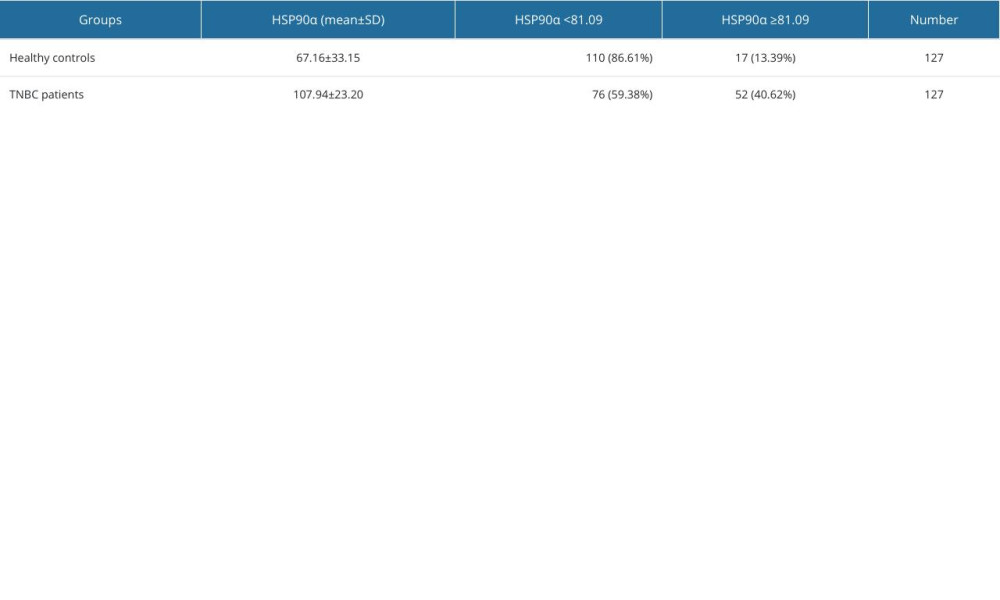 Table 2. Comparison of HSP90α value between patients with TNBC and healthy controls.
Table 2. Comparison of HSP90α value between patients with TNBC and healthy controls.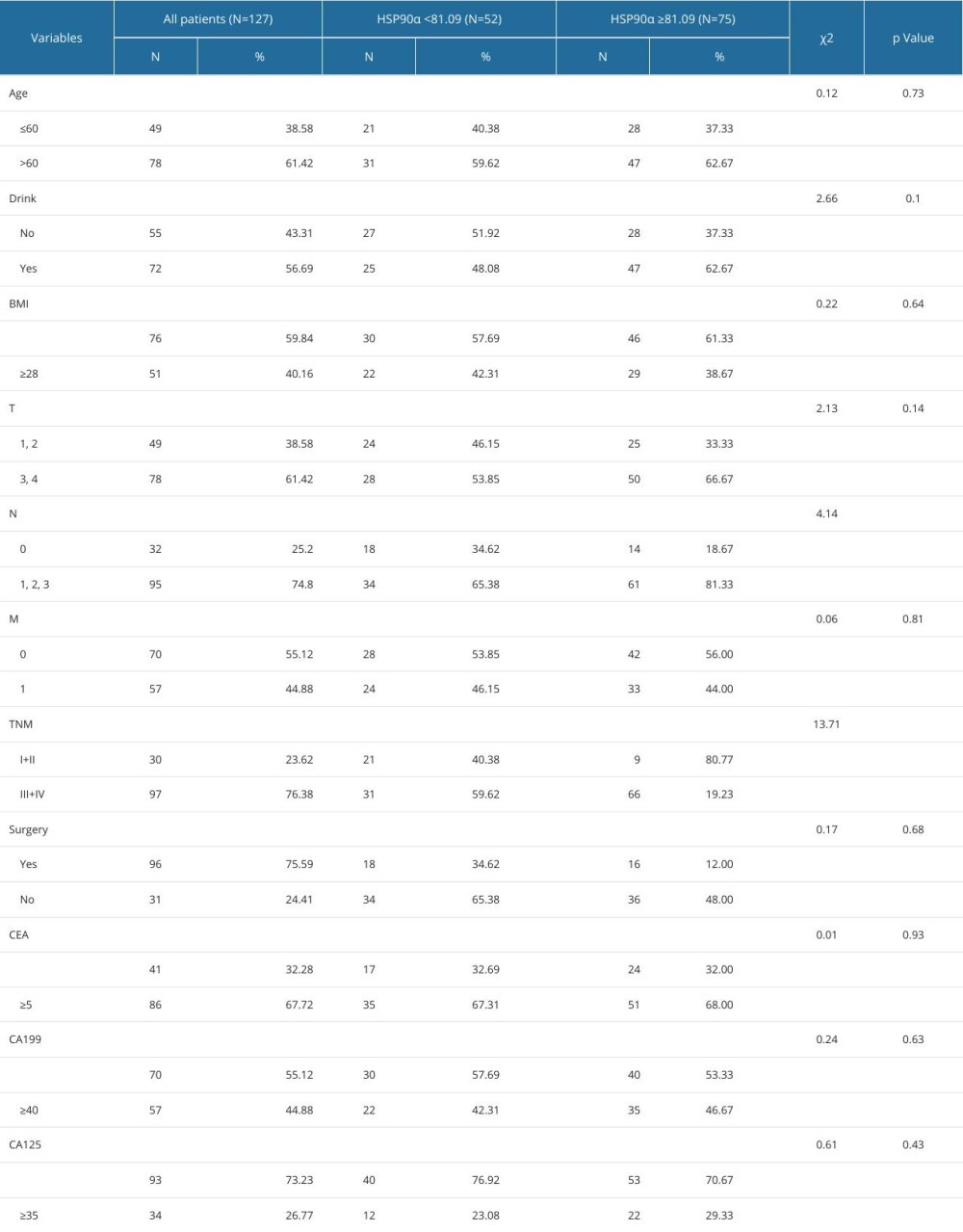 Table 3. Baseline characteristics in 128 patients with TNBC.
Table 3. Baseline characteristics in 128 patients with TNBC.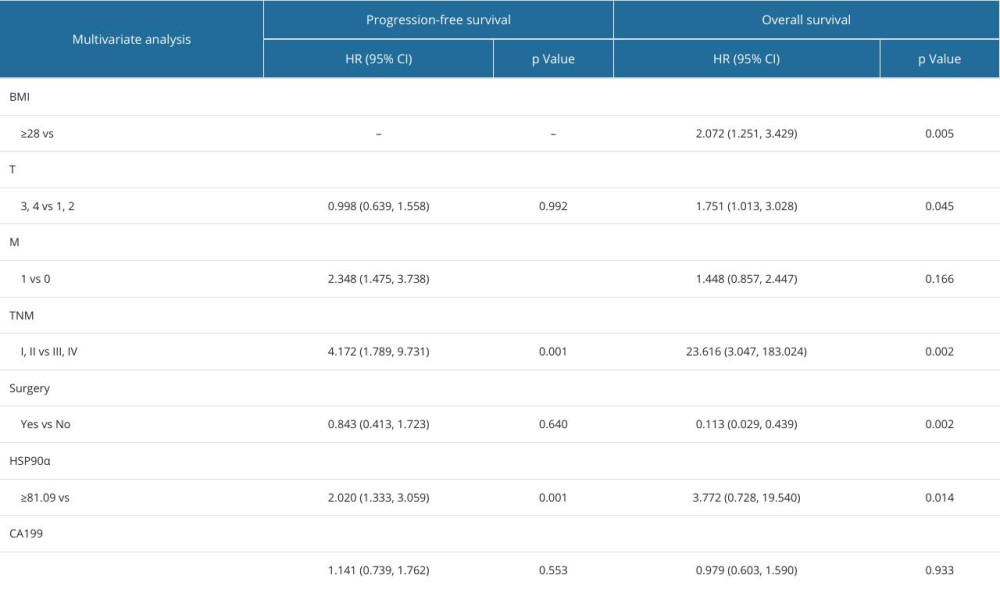 Table 4. Cox multivariate survival analyses of 127 patients with TNBC.
Table 4. Cox multivariate survival analyses of 127 patients with TNBC. Table 1. Cutoff value and OS diagnostic efficacy of HSP90α.
Table 1. Cutoff value and OS diagnostic efficacy of HSP90α. Table 2. Comparison of HSP90α value between patients with TNBC and healthy controls.
Table 2. Comparison of HSP90α value between patients with TNBC and healthy controls. Table 3. Baseline characteristics in 128 patients with TNBC.
Table 3. Baseline characteristics in 128 patients with TNBC. Table 4. Cox multivariate survival analyses of 127 patients with TNBC.
Table 4. Cox multivariate survival analyses of 127 patients with TNBC. In Press
08 Mar 2024 : Animal Research
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hyp...Med Sci Monit In Press; DOI: 10.12659/MSM.943443
18 Apr 2024 : Clinical Research
Comparative Analysis of Open and Closed Sphincterotomy for the Treatment of Chronic Anal Fissure: Safety an...Med Sci Monit In Press; DOI: 10.12659/MSM.944127
08 Mar 2024 : Laboratory Research
Evaluation of Retentive Strength of 50 Endodontically-Treated Single-Rooted Mandibular Second Premolars Res...Med Sci Monit In Press; DOI: 10.12659/MSM.944110
11 Mar 2024 : Clinical Research
Comparison of Effects of Sugammadex and Neostigmine on Postoperative Neuromuscular Blockade Recovery in Pat...Med Sci Monit In Press; DOI: 10.12659/MSM.942773
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








