28 April 2024: Animal Study
Modification of Experimental Model of Necrotizing Enterocolitis (NEC) in Rat Pups by Single Exposure to Hypothermia and Hypoxia and Impact of Mother’s Milk on Incidence of Disease
Marek WolskiDOI: 10.12659/MSM.943443
Med Sci Monit 2024; 30:e943443
Abstract
BACKGROUND: Necrotizing enterocolitis (NEC) is a potentially life-threatening disease that affects the intestine of the neonate, causing necrosis and general inflammation. Treatment consists of feeding cessation and antibiotic therapy. In more severe cases, surgical intervention is necessary. Recently, different NEC models have been used to study the development of novel diagnostic and therapeutic methods. This work modified an experimental NEC model in rat pups by a single exposure of animals to NEC-causing factors and testing the impact of mother’s milk on prevalence of the disease.
MATERIAL AND METHODS: Fifty rat pups were subjected to the NEC protocol, in which they were exposed to 100% nitrogen atmosphere and cold stress for set periods of time and formula feeding with exposure to mother’s milk and artificial milk. Twenty-nine pups were used for control. After a set time of 72 h, bowel fragments were obtained and examined histologically by hematoxylin-eosin staining with a modified 3-grade scale.
RESULTS: Histological features of NEC were present in most of the samples (10/14) in the group exposed to 1 min of hypoxia (P=0.016), 10 min of cold stress (P=0.4) and formula feeding every 3 h with no mother’s milk (P=0.001). In the group of 11 animals with the same stress conditions but fed mother’s milk right after birth, only 1 sample of NEC was present.
CONCLUSIONS: The modified experimental NEC model based on formula feeding and single exposure to hypothermia and hypoxia was assessed statistically and histologically. In this model, mother’s milk had a protective effect against necrotizing enterocolitis.
Keywords: Enterocolitis, Necrotizing, Disease Models, Animal, Breast Milk Expression
Introduction
NEC, NECROTIZING ENTEROCOLITIS:
NEC is a disease of newborns, leading to a generalized inflammatory process and bowel wall necrosis [6]. It primarily affects premature babies – the lower the birthweight, the greater the risk of NEC [2]. Morbidity and mortality are highest in newborns with birthweights below 1000 g [2]. The estimated incidence of NEC is 1 per 1000 newborns. In the group with very low birthweight, this figure goes up to 20% [3]. Mortality is estimated at 15–30%, with an inverse correlation of gestational age and birthweight to survival [2,3]. In newborns born at term, NEC is associated with risk factors like congenital defects and episodes of hypoxia [2].
Surgical treatment of NEC started in the 1940s [3]. Although NEC is the most common indication for surgical intervention in neonates, the pathogenesis remains unclear. The list of the most important presumed underlying mechanisms includes hypoxia, compromised intestinal perfusion, and bacterial colonization [2,3]. The risk factors are very low birthweight, early formula feeding, intestinal dysbiosis, prematurity, hypoxia, and congenital heart defects, and on the mother’s side include gestational infection, metabolic diseases, cocaine use, and hypoxia [2,3].
The treatment of NEC comprises medical and surgical approaches, with bowel perforation being an absolute indication for surgery [3,4,6]. Conservative treatment is based on wide-spectrum antibiotics, oral feeding cessation, and decompression of the digestive tract [2–4]. There is no single surgical strategy for NEC. The available options depend on the clinical condition of the patient and progression of bowel necrosis.
In terms of prophylaxis, oral feeding with breast milk has the strongest scientific evidence. There is still controversy over prophylactic use of probiotics [6].
DEVELOPMENT OF A RAT NEC MODEL:
Experimental research on NEC dates to 1975 and the seminal study published by Barbara Barlow and Thomas Santulli [7], showing that NEC-like changes in the bowel structure were caused by repeated episodes of hypothermia and hypoxia, and this was verified histologically. Since then, the model has been extensively used and modified. The basic principles, however, remains unchanged. Caplan et al tested this experimental setting with formula feeding and bacterial infection of the intestine [1,8]; hypoxia was found to be necessary to produce necrosis of the bowel. Nadler et al and Dvorak et al enriched the model with data on the protective role of the epithelial growth factor (EGF) and maternal milk, and demonstrated the correlation between NEC and levels of nitric oxide synthase (iNOS) and IL-12 [9,10]. They also proposed a histological grading scale of experimental NEC [9,10]. In the following years, other factors were tested with the use of similar models: mother’s cortisol, vitamin E, captopril, unsaturated fatty acids, pentoxifylline, granulocyte colony-stimulating factor, arginine, anti-TNF-alpha, epidermal growth factor, human breast milk oligosaccharides, probiotics, maternal N-acetylcysteine, antenatal dexamethasone, and surfactant [11–26].
The influence of mother’s milk was assessed in experimental models with newborn dogs, goats, pigs, and rats. Although it is hard to directly extrapolate the results to predict human outcomes, there is a consensus in the literature concerning the protective value of mother’s milk against inflammation in the intestine in comparison with formula feeding and complete feeding cessation [11,27].
AIM OF THE STUDY:
The aim of this study was to modify an experimental NEC model in rat pups by a single exposure of animals to NEC-causing factors and test the impact of mother’s milk feeding on the prevalence of the disease.
Unlike in other experiments based on the work of Barlow and Santulli, the idea here was to bring the NEC model as close as possible to the clinical setting. Instead of repeated exposures to hypoxia and hypothermia, the animals were exposed to the stress factors only once, at the beginning of the experiment, just like human newborns after birth during their transition to the Intensive Care Unit (ICU).
Material and Methods
ETHICS STATEMENT:
The experiment was approved by the 1st Local Ethics Committee for Animal Experiments in Warsaw (approval 161/2016) and conducted in the years 2018–2020. Seven adult and 79 newborn Sprague-Dawley rats were used in the experiment. The NEC protocol consisted of hypoxia, hypothermia, and formula feeding.
PRETERM LABOR:
Preterm labor was initially induced by subcutaneous administration of oxytocin on the 19th day of pregnancy, which was later dropped due to inability to obtain live rat pups. The control group consisted of 29 puppies that were killed right after birth.
HYPOTHERMIA-HYPOXIA-FORMULA FEEDING:
Fifty newborn rats, divided in subgroups 1–6, were put in individual ventilated cages (IVC) and subjected to 100% nitrogen atmosphere for 0.5 or 1 min and temperature of 4°C for 5 or 10 min. After a set time, the cages were placed in an environment at 37°C and the animals were fed formula in standard dilution using a 0.5-mm gauge feeder tube every 3 h during the experiment, or were fed mother’s milk immediately after birth and then were fed formula every 3 h.
EUTHANASIA:
After 72 h, the animals were euthanized and small and large intestine samples were collected for histological assessment with hematoxylin-eosin staining (Figure 1). Twenty-nine newborn pups from oxytocin-induced labor were used as a histological control group.
HISTOLOGICAL ASSESSMENT:
Each specimen was fixed in 10% neutral buffered formalin solution. Fixed samples were embedded in paraffin, cut into 3-μm sections, and stained routinely with hematoxylin-eosin (HE) for morphological examination.
Slides were first assessed at low magnification (40×) to evaluate the general architecture of the colon. Next, slides were viewed at 200× and 400× and assessed semiquantitatively in a 3-class scoring system as: grade 0 (no change in the bowel wall), grade 1 (submucosal and lamina propria separation with edema of the submucosa), and grade 2 (total loss of villi and total necrosis of the intestinal wall) (Table 1, Figure 2).
The most representative slides were digitized with a Hamamatsu NanoZoomer 2.0-HT scanner (Hamamatsu, Japan). Microphotographs were captured using NDP.view2 software (Hamamatsu, Japan).
STATISTICAL ANALYSIS:
Fifty rats were tested, having been subjected to NEC-causing factors. They were (n=23) or were not (n=27) given mother’s milk immediately after birth. The duration of hypothermia was 5 or 10 min and hypoxia lasted 30 or 60 s. Hypothermia, hypoxia, and mother’s milk were the independent variables in the model. The finding of NEC in bowel sample histology was the dependent variable. Logistic regression was used to build a statistical model of the disease. The parameters are presented in Table 2. Variables hypoxia and mother’s milk were found significant for the model, with P values of 0.016 and 0.001, respectively. Although the P value for hypothermia was 0.4 and was not significant, it did not affect the accuracy of the model, which was found to be well fitted to the data based on the Hosmer–Lemeshow test and the Nagelkerke coefficient of determination.
Results
PRETERM BIRTH:
Preterm birth was provoked by administering 1 IU of oxytocin subcutaneously in the 3 first litters, but all pups (n=29) died immediately after birth. Then, the oxytocin administration was excluded from the protocol and the dead pups from the first 3 litters were used as a control group for histopathological assessment.
NEC MODEL:
Pups born in consecutive litters (n=50, Table 3) were included in the NEC protocol and were divided into 6 groups. Each of these groups was exposed to NEC-causing factors of differing intensity. In groups 1 and 2 the period of hypoxia was set at 30 s and hypothermia at 5 and 10 min, respectively. NEC was obtained in 2 specimens from group 1 and no specimens from group 2. In groups 3 and 4, the period of hypoxia was set at 60 s and hypothermia was set at 5 and 10 min, respectively. The pups from groups 3 and 4 were fed mother’s milk immediately after birth. No NEC-positive specimens were obtained in groups 3 and 4. Group 5 and 6 were submitted to the highest-intensity factors – 1 min of hypoxia and 10 min of hypothermia. The pups from group 5, unlike those from group 6, were dam-fed once immediately after birth. NEC was obtained in 1 and 10 specimens from groups 5 and 6, respectively. The results are presented in Table 3.
Discussion
The present study modified the hypothermia-hypoxia NEC model in newborn rats by single exposure to disease-causing factors and tested the positive effect of being fed mother’s milk on the prevalence of NEC. In the modified model, a 1-min period of hypoxia in 100% nitrogen atmosphere and hypothermia at 4°C for 10 min followed by formula feeding every 3 h resulted in histologically confirmed NEC in 10 out of 14 newborns. In the same modelling conditions, newborns given mother’s milk right after birth developed NEC in only 1 out of 11 cases.
A well-designed and scientifically reliable model of a disease should have several characteristics. According to Sodhi et al, an animal NEC model should mimic the pathophysiological mechanism of necrotizing enterocolitis and present a similar histological appearance [1]. Meeting these requirements is a considerable challenge, especially as the pathophysiology of NEC is not well established. The main criteria quoted in the literature are: newborn status of animals, prematurity, onset of disease after the beginning of oral feeding, and no predilection for any specific segments of the intestine [1].
Summarizing the literature on the use of different animal species in NEC modelling, Sibbons et al found that most of the research has produced data that are hard to interpret and implement, mostly due to the invalid construction of experiments [11], with the main flaws being inadequate choice of animals, use of animals beyond the neonatal period, and clinically irrelevant NEC-causing factors.
Induction of NEC using factors that are not relevant in the clinical setting is controversial. Cohen and Nelson, for example, apart from hypothermia and hypoxia, used a mechanical tonometer to increase the pressure in the rectal bulb and cause NEC-like changes in the bowel of guinea pigs [28]. They reported a high rate of complications that accompany the use of a mechanical factor. A substantial amount of research on NEC has been dedicated to the occlusion of mesenteric vessels. Sibbons and Spitz found a whole spectrum of NEC-like changes following mechanical occlusion of mesenteric vessels in piglets [29,30]. Intestinal necrosis caused by a mesenteric vessel occlusion enables histological analysis of the patterns of bowel wall necrosis. Mechanical pressure or chemical irritation of the intestinal mucosa eventually cause inflammation and necrosis, as does recurrent exposure to hypothermia and hypoxia, but all these are far from the actual clinical context leading to NEC.
The choice of a particular animal species for the experiment in this context requires that it allow for extrapolation of the results to humans. Sibbons concluded that the animals used in well-designed research studies are predominantly pigs, rats, rabbits, and dogs, and concluded that the pig model is the closest to the real-life setting [11]. Numerous studies based on the rat model have shown the possibility of causing histopathological changes characteristic of NEC using factors closely resembling the clinical setting. The inflammatory profile of rat NEC seems to be compliant with the human NEC, as shown by Dvorak, with the dominant role of interleukins Il-12 and Il-18 [31]. Another observation adding to the high value of the rat NEC model is the impact of being fed mother’s milk and maternal milk consumption in general on reducing the incidence and severity of the disease, which aligns with the clinical picture of NEC and experimental observations of other authors (eg, Dvorak and Sodhi) [31]. The mouse models, being relatively easily accessible and reproducible, offer the opportunity for genetic modification to study the pathogenesis of the disease. [5]
The NEC-causing factors used in the present study do not differ from the standard experimental setting described and tested over the past 30 years of NEC modelling. Hypoxia and hypothermia together with formula feeding were used by Barlow and Santulli in their original experimental study and subsequently modified multiple times by other authors [7]. Uniquely, the model they presented differs in terms of timing and frequency of the application of hypoxia and hypothermia – they were applied only once, immediately after birth. Formula feeding was the only stimulus repeated every 3 h throughout the experiment. Originally, the use of preterm pups was planned. This experimental setting was supposed to provide the closest possible resemblance to the clinical conditions, and it had not been tested or reported before.
With respect to the age of the animals used, there is a consensus that they should be enrolled in the protocol immediately after birth. Sodhi et al designed a reproducible murine NEC model that proved it was impossible to induce the disease with the same agents in animals older than 14 days [1]. Animal NEC models based on adult animals have a limited capacity for producing high-quality evidence [11]. There is some controversy surrounding the prematurity factor; depending on the species, the features of prematurely and term-born animals differ largely. As postulated by Dvorak et al, based on Pacha et al, the length of gestation is the deciding factor [32]. In mammals with long gestation periods, like humans, pigs, and sheep, and unlike rodents, most of the growth and maturation of the bowel takes place in utero. In the case of these animals, it would be reasonable to use prematurely-born pups. Sibbons et al showed a minimal response to NEC-causing factors in term-born piglets, and the same factors were very effective in the model based on premature animals [33]. In species with short gestation periods, the final growth and maturation of the gastrointestinal tract takes place after birth [32]. This warrants the assumption that the gastrointestinal tract of a term-born rat corresponds with the gastrointestinal tract of a preterm human newborn [31]. Regardless of the arguments quoted, most authors emphasize prematurity. Different authors describe various techniques of acquiring premature rat and mouse pups. Zhou et al performed a C-section on day 22 of gestation [34]. Gonçalves et al described a method for inducing labor with 1 Ul of oxytocin injected subcutaneously [35]. In the primary experimental setting of the present model, it was decided to acquire premature pups using the method described by Gonçalves. However, in 3 consecutive trials with model characteristics meeting the description in the research paper, it was impossible to obtain living pups. All animals were either stillborn or died immediately after birth. Due to the inability to enroll animals in the protocol and the restrictions of the local ethics committee, at this point the decision was made to set aside the prematurity criterion. The decision was based on the arguments mentioned above: that the rodent NEC model based on mature pups meets the quality requirements. The final version of the model following the above-mentioned reasoning was built on variables mirroring the clinical setting of the intensive care unit: hypoxia, hypothermia, formula feeding.
The IVC cage (individual, ventilated) used in the experiment allowed control over the amount of oxygen in the atmosphere in a given time period. As a result of controlled hypoxia and change in the blood perfusion pattern, the bowel wall is affected by ischemia. The length of hypoxia of 30 s and 60 s was adopted following literature analysis, mainly relying on the work of Barlow and Santulli and the model verification by Gonçalves et al, with modifications planned throughout the experiment if necessary [7,35].
The amount of observed ischemic changes to the bowel wall increased with the length of hypoxia. This was in line with the observations of Barlow and Santulli, where the amount of inflammatory and necrotic changes rose with the number of hypoxic episodes [7]. In that experiment, as well as in all settings based on it, the effect of NEC-like changes in the bowel wall was achieved by repeated exposure to hypoxia and hypothermia [1,7,11,36]. To better mimic the clinical setting in the present experiment, newborn rats were exposed to hypoxia and hypothermia only once – immediately after birth. With artificial formula feeding, this was sufficient to induce histologically confirmed NEC.
In 1974, Toubas et al proved experimentally that hypothermia restricts the blood flow through most of the internal organs in newborn sheep [37]. Barlow and Santulli showed the same effect of exposure to 10 min of hypothermia together with hypoxia and artificial feeding [7]. In the present experiment, newborn rats were exposed to hypothermia at 4°C for 5 and 10 min. Most of the histological changes in the bowel were present in the 10-min hypothermia group. This stressor was used only once, immediately after birth, as was hypoxia.
A separate subgroup of animals consisted of pups that drank mother’s milk before being separated from the mother; in the entire group, NEC was observed in the histological bowel examination of only 1 rat. These observations are consistent with the results published by Dvorak et al, who found no cases of NEC in dam-fed animals as opposed to those fed artificially and to a lesser extent those bottle-fed with maternal milk. The authors suggested a possible mechanism responsible for this effect – increased level of the anti-inflammatory cytokine IL-10 and decreased levels of pro-inflammatory IL12 and IL-18 [32].
Histological assessment served to verify the assumptions of the NEC model. The initial assessment was conducted according to the scale proposed by Dvorak et al [10], which consists of 4 grades representing the progression of the disease and grade 0, which indicates a normal bowel wall. In the histological analysis of specimens, the present study could not observe the changes to the bowel wall proposed in Dvorak’s scale, which was found it to be very subjective; for example, it is difficult to differentiate between mild, moderate, and severe separation of the lamina propria. In the present model, the prevalent histological appearance (n=13) was near-total necrosis (grades III/IV according to Dvorak), with only 1 animal showing mild NEC-like changes, so it was decided to create a simplified 3-level scale tailored to the needs of this particular disease model. The levels were: I (normal intestine), II (submucosal and lamina propria separation with edema of the submucosa), and III (villi loss and necrosis).
The main limitation of the present study was the inability to acquire premature rat pups as originally planned. Even though it does not affect the efficacy of the model in obtaining NEC, it limits the control over the beginning of the experimental trial, as the pups in different groups were born on days 19 to 23 of pregnancy. Also, the confirmation of NEC only by histopathological hematoxylin-eosin assessment might be insufficient depending on the future application of the model. Further methods, such as immunohistochemical analyses of different cells or biochemical analyses of the peritoneal fluid, should be applied depending on the desired results of the experiment and possible diagnostic or therapeutic methods tested.
Conclusions
The modified experimental NEC model based on formula feeding and single exposure to hypothermia and hypoxia was verified statistically and histologically. In this model, being fed mother’s milk protected against necrotizing enterocolitis.
Figures
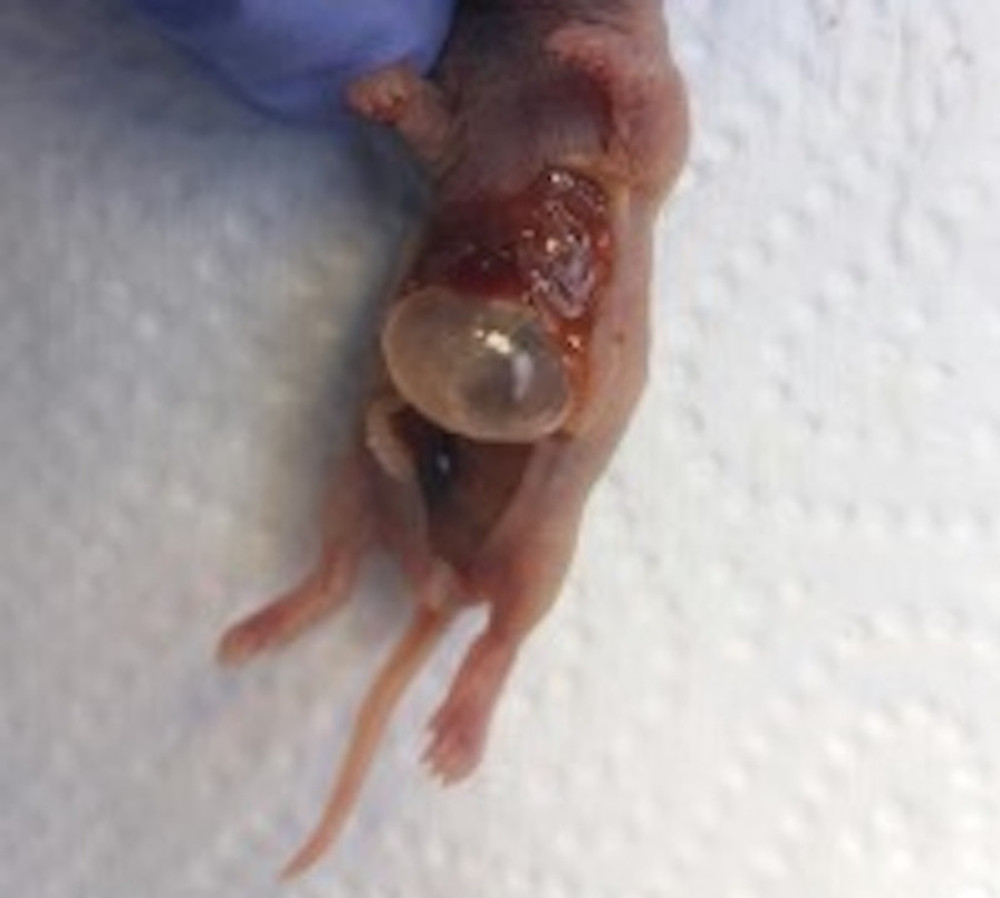 Figure 1. Newborn rat euthanized after 72 h of NEC model duration, before intestine collection. Source: Own materials.
Figure 1. Newborn rat euthanized after 72 h of NEC model duration, before intestine collection. Source: Own materials. 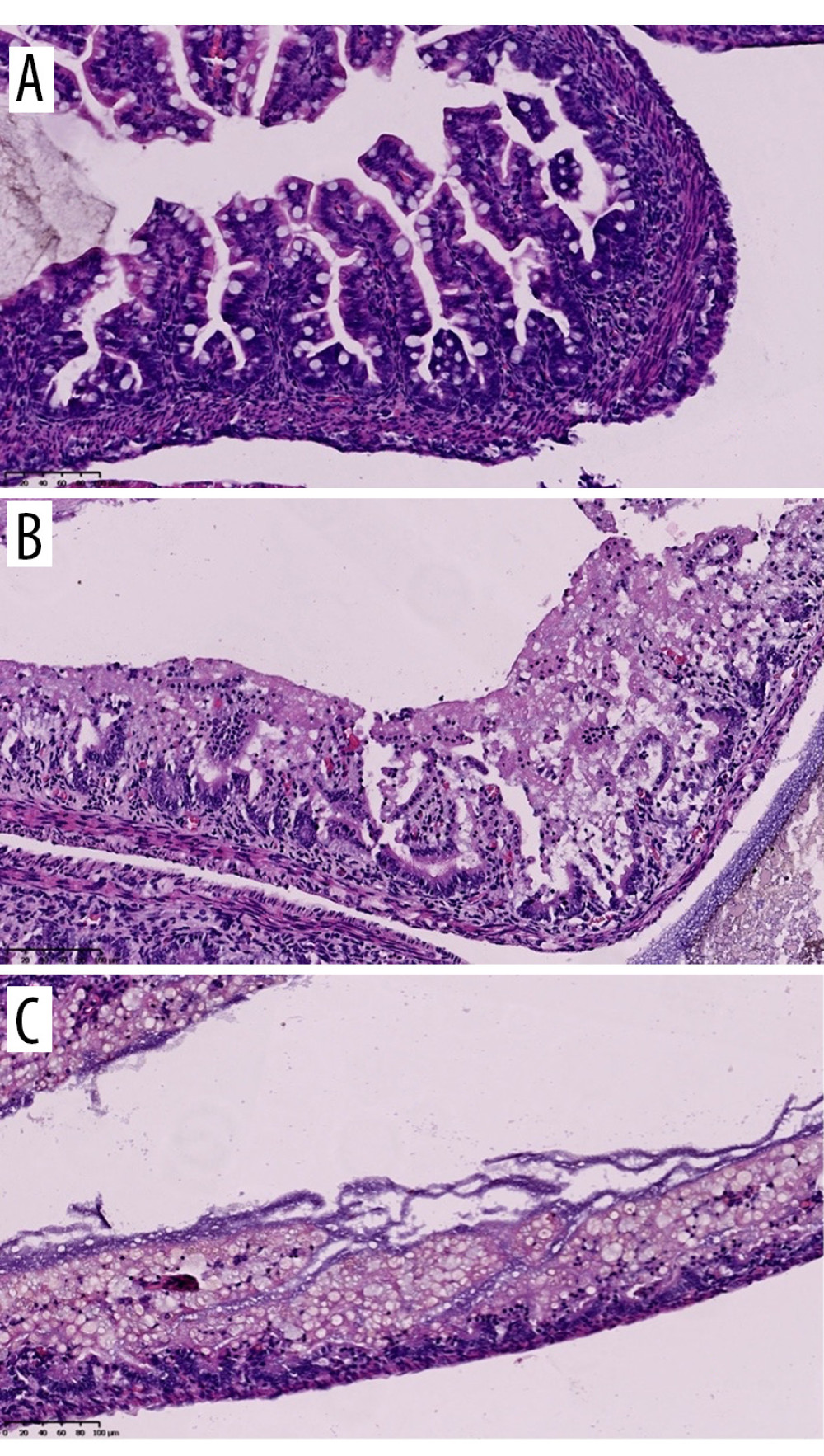 Figure 2. Histology: hematoxylin-eosin (HE) staining. Samples of the small intestine representing different levels of the NEC grading scale. A: grade 0 – normal intestine, B: grade I – submucosal and lamina propria separation with oedema of the submucosa, C: grade II – villi loss and necrosis. Source: Own materials, NDP.view2 software (Hamamatsu, Japan).
Figure 2. Histology: hematoxylin-eosin (HE) staining. Samples of the small intestine representing different levels of the NEC grading scale. A: grade 0 – normal intestine, B: grade I – submucosal and lamina propria separation with oedema of the submucosa, C: grade II – villi loss and necrosis. Source: Own materials, NDP.view2 software (Hamamatsu, Japan). Tables
Table 1. NEC (necrotizing enterocolitis) grading scale used in the experiment. Table 2. Statistical verification of the NEC model. Logistic regression model parameters with standard deviation and P values for each variable.
Table 2. Statistical verification of the NEC model. Logistic regression model parameters with standard deviation and P values for each variable.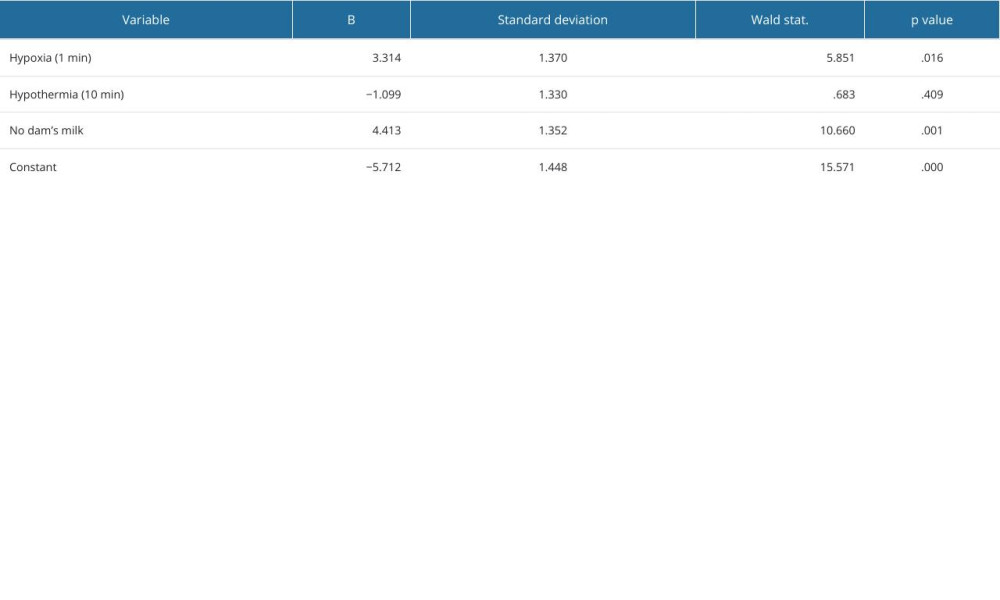 Table 3. Results of the experiment. In rows representing subgroups of animals, different intensities of the variables (hypothermia, hypoxia, dam milk) and number of obtained NEC samples are quoted, next to the number of animals in each subgroup and the day of labor.
Table 3. Results of the experiment. In rows representing subgroups of animals, different intensities of the variables (hypothermia, hypoxia, dam milk) and number of obtained NEC samples are quoted, next to the number of animals in each subgroup and the day of labor.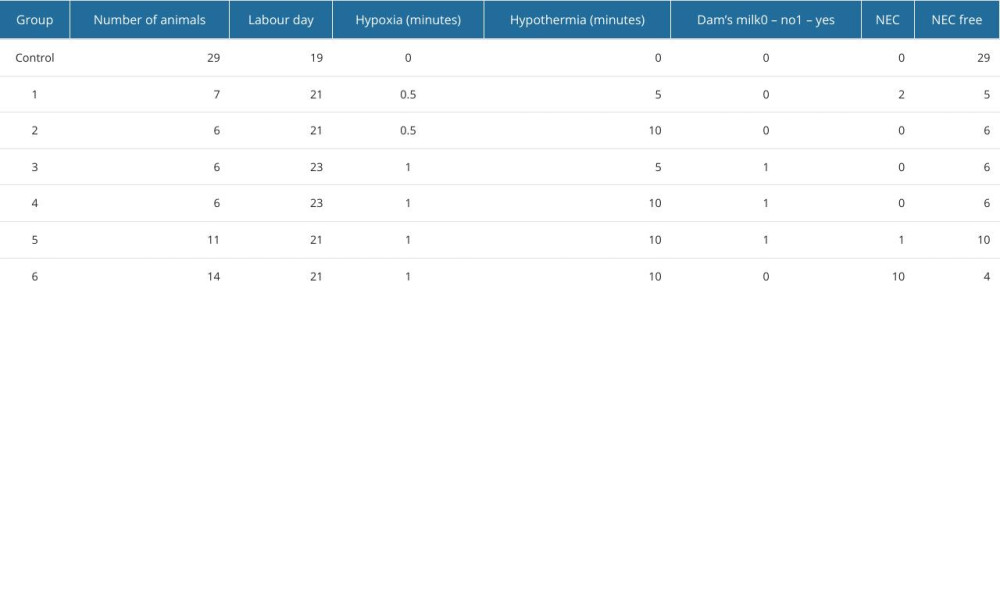
References
1. Sodhi C, Richardson W, Gribar S, Hackam DJ, The development of animal models for the study of necrotizing enterocolitis: Dis Model Mech, 2008; 1(2–3); 94-98
2. Lawrence Moss R, Necrotising enterocolitis: Ashcraft’s pediatric surgery, 2010; 439-55, Philadelphia, Saunders Necrotizing Enterocolitis
3. Alganabi M, Lee C, Bindi E, Recent advances in understanding necrotizing enterocolitis: F1000Research, 2019; 8 F1000 Faculty Rev-107
4. Ginglen JG, Butki N, Necrotizing enterocolitis. [Updated 2023 Aug 8]: StatPearls [Internet], 2024, Treasure Island (FL), StatPearls Publishing Available from: https://www.ncbi.nlm.nih.gov/books/NBK513357/
5. Lopez CM, Sampah MES, Duess JW, Models of necrotizing enterocolitis: Semina Perinatol, 2023; 47; 151695
6. Neu J, Necrotising enterocolitis: The future: Neonatology, 2020; 117; 240-44
7. Barlow B, Santulli TV, Importance of multiple episodes of hypoxia or cold stress on the development of enterocolitis in an animal model: Surgery, 1975; 5; 687-90
8. Caplan M, Miller-Catchpole R, Kaup S, Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model: Gastroenterology, 1999; 117; 577-83
9. Nadler EP, Dickinson E, Knisely A, Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis: J Surg Res, 2000; 92(1); 71-77
10. Dvorak B, Halpern MD, Holubec H, Epidermal growth factor reduces the development of necrotizing enterocolitis in a neonatal rat model: Am J Physiol Gastrointest Liver Physiol, 2002; 282(1); G156-64
11. Sibbons P, Spitz L, van Velzen D, The use of animal models in the study of necrotizing enterocolitis in the newborn: Semin Neonatol, 1997; 2; 281-90
12. Seitz G, Warmann SW, Guglielmetti A, Protective effect of tumor necrosis factor alpha antibody on experimental necrotizing enterocolitis in the rat: J Pediatr Surg, 2005; 40(9); 1440-45
13. Cetin S, Leaphart CL, Ischenko I, Nitric oxide inhibits enterocyte migration through activation of RhoA-GTPase in a SHP-2-dependent manner: Am J Physiol, 2007; 292; G1347-58
14. Israel EJ, Schiffrin EJ, Carter EA, Prevention of necrotizing enterocolitis in the rat with prenatal cortisone: Gastroenterology, 1990; 99(5); 1333-38
15. Cadir FO, Bicakci U, Tander B, Protective effects of vitamin E and omeprazole on the hypoxia/reoxygenation induced intestinal injury in newborn rats: Pediatr Surg Int, 2008; 24; 809-13
16. Zani A, Eaton S, Leon FF, Captopril reduces the severity of bowel damage in a neonatal rat model of necrotizing enterocolitis: J Pediatr Surg, 2008; 43(2); 308-14
17. Lu J, Jilling T, Li D, Polyunsaturated fatty acid supplementation alters proinflammatory gene expression and reduces the incidence of necrotizing enterocolitis in a neonatal rat model: Pediatr Res, 2007; 61; 427-32
18. Travadi J, Patole S, Charles A, Pentoxifylline reduces the incidence and severity of necrotizing enterocolitis in a neonatal rat model: Pediatr Res, 2006; 60; 185-89
19. Canpolat FE, Yurdakok M, Ozsoy S, Protective effects of recombinant human granulocyte colony stimulating factor in a rat model of necrotizing enterocolitis: Pediatr Surg Int, 2006; 22; 719-23
20. Halpern MD, Clark J, Saunders TA, Reduction of experimental necrotizing enterocolitis with anti-TNF-alpha: Am J Physiol, 2006; 290; G757-64
21. Wenqian C, Changyi Heng YX, The protective effect and mechanism of epidermal growth factor on necrotizing enterocolitis in a neonatal rat model: Transl Pediatr, 2021; 10(4); 900-13
22. He-Yang J, Zhang W, Liu J, Human breast milk oligosaccharides attenuate necrotizing enterocolitis in rats by suppressing mast cell accumulation, DPPI activity and TLR4 expression in ileum tissue, and regulating mitochondrial damage of Caco-2 cells: Int Immunopharmacol, 2020; 88; 106881
23. Lin X, Wu C: PLoS One, 2023; 18(11); e0287799
24. Zmora O, Gutzeit O, Segal L, Maternal N-acetyl-cysteine prevents neonatal brain injury associated with necrotizing enterocolitis in a rat model: Acta Obstet Gynecol Scand, 2021; 100(5); 979-87
25. Beloosesky R, Gutzeit O, Ginsberg Y, Intestine and brain TLR-4 modulation following N-acetyl-cysteine treatment in NEC rodent model: Sci Rep, 2023; 13(1); 8241
26. Lu L, Lu J, Yu Y, Claud E, Necrotizing enterocolitis intestinal barrier function protection by antenatal dexamethasone and surfactant-D in a rat model: Pediatr Res, 2021; 90(4); 768-75
27. Barlow B, Santulli SV, Heird WC, An experimental study of acute necrotizing enterocolitis – the importance of breast milk: J Pediatr Surg, 1974; 9; 587-95
28. Cohen IT, Nelson SD, Moxeley RA, Necrotising enterocolitis in a neonatal piglet model: J Pediatr Surg, 1991; 26; 598-601
29. Sibbons PD, Spitz L, van Velzen D, Necrotizing enterocolitis induced by local circulatory interruption in the ileum of neonatal piglets: Pediatr Path, 1992; 12; 1-14
30. Sibbons PD, Spitz L, van Velzen D, Collateral blood flow in the distal ileum of neonatal piglets: A clue to the pathogenesis of necrotizing enterocolitis: Pediatr Path, 1992; 12; 15-27
31. Dvorak B, Halpern M, Holubec H, Maternal milk reduces severity of necrotizing enterocolitis and increases intestinal IL-10 in a neonatal rat model: Pediatr Res, 2003; 53; 426-33
32. Pacha J, Development of intestinal transport function in mammals: Physiol Rev, 2000; 80; 1633-77
33. Sibbons PD, Spitz L, van Velzen D, Relationship of birth-weight to the pathogenesis of necrotizing enterocolitis in the neonatal piglet: Pediatr Path, 1988; 8; 151-62
34. Zhou W, Zheng XH, Rong X, Establishment and evaluation of three necrotizing enterocolitis models in premature rats: Mol Med Rep, 2011; 4; 1333-38
35. Gonçalves FLL, Gallindo RM, Soares LMM, Validation of protocol of experimental necrotizing enterocolitis in rats and the pitfalls during the procedure: Acta Cir Bras, 2013; 28(1); 2013-19
36. Caplan MS, Sun XM, Hsueh W, Hypoxia causes ischemic bowel necrosis in rats: the role of platelet-activating-factor (PAF-acether): Gastroenterology, 1990; 99; 979-86
37. Toubas PL, Hof PR, Heymann HA, Effects of hypothermia and rewarming on neonatal circulation: Pediatr Res, 1974; 8; 451-59
Figures
 Figure 1. Newborn rat euthanized after 72 h of NEC model duration, before intestine collection. Source: Own materials.
Figure 1. Newborn rat euthanized after 72 h of NEC model duration, before intestine collection. Source: Own materials. Figure 2. Histology: hematoxylin-eosin (HE) staining. Samples of the small intestine representing different levels of the NEC grading scale. A: grade 0 – normal intestine, B: grade I – submucosal and lamina propria separation with oedema of the submucosa, C: grade II – villi loss and necrosis. Source: Own materials, NDP.view2 software (Hamamatsu, Japan).
Figure 2. Histology: hematoxylin-eosin (HE) staining. Samples of the small intestine representing different levels of the NEC grading scale. A: grade 0 – normal intestine, B: grade I – submucosal and lamina propria separation with oedema of the submucosa, C: grade II – villi loss and necrosis. Source: Own materials, NDP.view2 software (Hamamatsu, Japan). Tables
 Table 1. NEC (necrotizing enterocolitis) grading scale used in the experiment.
Table 1. NEC (necrotizing enterocolitis) grading scale used in the experiment. Table 2. Statistical verification of the NEC model. Logistic regression model parameters with standard deviation and P values for each variable.
Table 2. Statistical verification of the NEC model. Logistic regression model parameters with standard deviation and P values for each variable. Table 3. Results of the experiment. In rows representing subgroups of animals, different intensities of the variables (hypothermia, hypoxia, dam milk) and number of obtained NEC samples are quoted, next to the number of animals in each subgroup and the day of labor.
Table 3. Results of the experiment. In rows representing subgroups of animals, different intensities of the variables (hypothermia, hypoxia, dam milk) and number of obtained NEC samples are quoted, next to the number of animals in each subgroup and the day of labor. Table 1. NEC (necrotizing enterocolitis) grading scale used in the experiment.
Table 1. NEC (necrotizing enterocolitis) grading scale used in the experiment. Table 2. Statistical verification of the NEC model. Logistic regression model parameters with standard deviation and P values for each variable.
Table 2. Statistical verification of the NEC model. Logistic regression model parameters with standard deviation and P values for each variable. Table 3. Results of the experiment. In rows representing subgroups of animals, different intensities of the variables (hypothermia, hypoxia, dam milk) and number of obtained NEC samples are quoted, next to the number of animals in each subgroup and the day of labor.
Table 3. Results of the experiment. In rows representing subgroups of animals, different intensities of the variables (hypothermia, hypoxia, dam milk) and number of obtained NEC samples are quoted, next to the number of animals in each subgroup and the day of labor. In Press
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
12 Mar 2024 : Review article
Optimizing Behçet Uveitis Management: A Review of Personalized Immunosuppressive StrategiesMed Sci Monit In Press; DOI: 10.12659/MSM.943240
12 Mar 2024 : Clinical Research
Metabolomic Alterations in Methotrexate Treatment of Moderate-to-Severe PsoriasisMed Sci Monit In Press; DOI: 10.12659/MSM.943360
14 Mar 2024 : Clinical Research
Renal Dysfunction Increases Risk of Adverse Cardiovascular Events in 5-Year Follow-Up Study of Intermediate...Med Sci Monit In Press; DOI: 10.12659/MSM.943956
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








