12 May 2024: Clinical Research
Evaluation of an Optimized Workflow for the Radiofrequency Catheter Ablation of Paroxysmal Atrial Fibrillation
Luqian Cui1ABCDEF, Shihua Cui2BE, Jingchao Li1CE, Haijia Yu1BE, Huihui Song1BE, Yingjie Chu1AEG, Shujuan Dong1AE*DOI: 10.12659/MSM.943526
Med Sci Monit 2024; 30:e943526
Abstract
BACKGROUND: A significant number of atrial fibrillation (AF) recurrences occur after initial ablation, often due to pulmonary vein reconnections or triggers from non-pulmonary veins.
MATERIAL AND METHODS: Patients with paroxysmal AF who underwent radiofrequency catheter ablation for the first time were enrolled. Base on propensity score matching (1: 1 matching), 118 patients were selected for an optimized workflow for the radiofrequency catheter ablation of paroxysmal AF (OWCA) group and a conventional group. Comparative analysis of the acute and 12-month clinical outcomes was conducted. Moreover, an artificial intelligence analytics platform was used to evaluate the quality of pulmonary vein isolation (PVI) circles.
RESULTS: PVI was successfully achieved in all patients. Incidence of first-pass isolation of bilateral PVI circles was higher (P=0.009) and acute pulmonary vein reconnections was lower (P=0.027) in the OWCA group than conventional group. The OWCA group displayed a significant reduction in the number of fractured points (P<0.001), stacked points (P=0.003), and a greater proportion of cases in which the radiofrequency index achieved the target value (P=0.003). Additionally, the contact force consistently met the force over time criteria (P<0.001) for bilateral PVI circles in the OWCA group, accompanied by a shorter operation time (P=0.017). During the 12-month follow-up period, the OWCA group exhibited a higher atrial arrhythmia-free survival rate following the initial ablation procedure than did the conventional group.
CONCLUSIONS: The optimized workflow for radiofrequency catheter ablation of paroxysmal AF could play a crucial role in creating higher quality PVI circles. This improvement is reflected in a significantly elevated 12-month atrial arrhythmia-free survival rate.
Keywords: Atrial Fibrillation, Catheter Ablation
Introduction
Clinical trials have demonstrated that performing radiofrequency catheter ablation (RFCA) for atrial fibrillation (AF) is a superior alternative to antiarrhythmic drugs for the maintenance of sinus rhythm, reduction of arrhythmia-related symptoms, and improvement of left ventricular ejection fraction (LVEF), all while having a comparable safety profile [1–3]. Therefore, RFCA has become the mainstream treatment for preventing AF recurrences [4]. Nevertheless, despite its established efficacy, a significant number of AF recurrences following initial ablation procedure can still occur due to factors such as pulmonary vein reconnections (PVRs) or triggers originating from non-pulmonary veins (PVs) [5–7].
In recent years, to improve the clinical outcomes following initial ablation procedure, various advancements in techniques and technologies have been introduced. These include the use of contact force-sensing catheters, an ablation index-guided high-power ablation strategy, steerable guiding sheaths, intracardiac echocardiography catheters, and the incorporation of superior vena cava isolation (SVCI) [8–17]. However, there is no standardized workflow in place, which can limit the effectiveness of AF ablation when performed by physicians in different medical centers.
The present study aimed to investigate the effectiveness of an optimized workflow for the RFCA of paroxysmal AF (OWCA) to generate contiguous and transmural lesions and to evaluate the acute and 12-month clinical outcomes associated with implementing this optimized workflow.
Material and Methods
STUDY PROTOCOL:
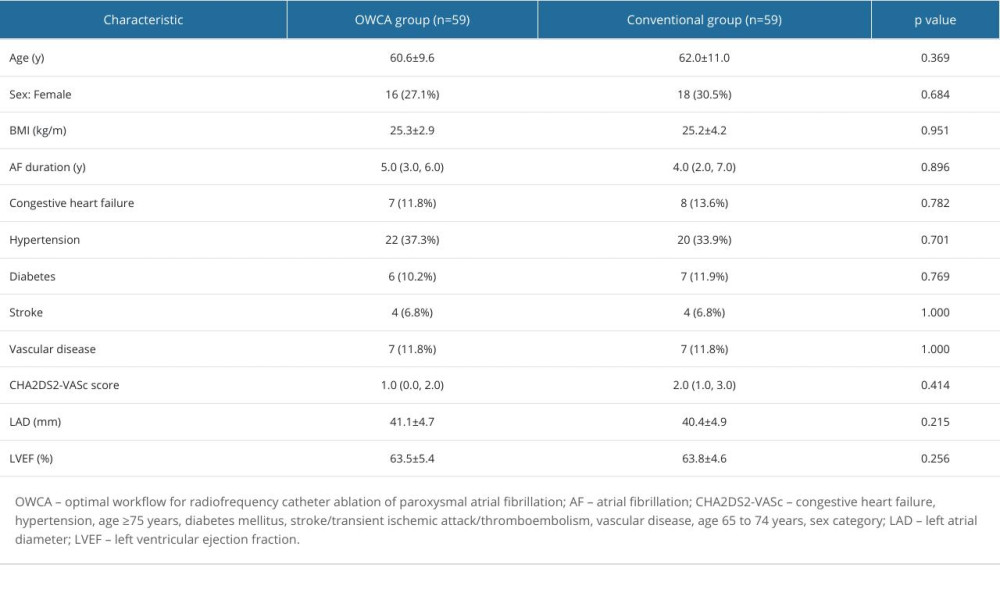
This study enrolled patients with paroxysmal AF who underwent RFCA for the first time between January 2019 and November 2021. Inclusion criteria were as follows: (1) RFCA performed for the first time; and (2) palpitation, chest tightness, and other symptoms that could not be controlled by antiarrhythmic drugs. Exclusion criteria were as follows: (1) thrombosis in the left atrium; (2) abnormal thyroid function; and (3) severe valvular heart disease or postoperative prosthetic valve surgery. Patients who adhered to the optimized workflow were defined as the OWCA group, and patients who followed the conventional workflow were designated as the conventional group. All procedures were performed by the same experienced operator. Propensity score matching of 1: 1 using age, sex, AF duration, CHA2DS2-VASc score, and left atrium diameter was conducted to control for selection bias, within a caliper of 0.05 on the propensity score.
All patients underwent cardiac contrast-enhanced computed tomography or transesophageal echocardiography to rule out left atrial thrombosis. All patients discontinued the use of antiarrhythmic drugs for more than 5 half-lives and used warfarin or new oral anticoagulants for a minimum of 4 weeks. All patients made their own choices in the ablation workflow and supplied their signed informed consent prior to any operations.
WORKFLOW OF OWCA GROUP:
Prior to the administration of anesthesia, all patients were connected to an automated external defibrillator to facilitate the conversion of AF to sinus rhythm. General anesthesia was subsequently administered by anesthesiologists to all patients. To aid in catheter and needle placement during the transseptal puncture procedure, an intracardiac echocardiography catheter (Soundstar; Biosense Webster, Irvine, CA, USA) was inserted into the right atrium. This catheter also served the purpose of monitoring the pericardial space in the event of a drop in oxygen saturation or arterial pressure during the procedure. A steerable decapolar catheter (DecaNAV; Biosense) was used to construct the matrix and was advanced into the coronary sinus.
Two transseptal punctures were meticulously performed at the optimal location on the interatrial septum using a Swartz sheath (Abbott, Chicago, IL, USA) and steerable sheath (VIZIGO; Biosense), respectively. These procedures were performed under X-ray and intracardiac echocardiographic guidance. After the first transseptal puncture, heparin was given at a dose of 100 U/kg body weight, with subsequent doses given to maintain the activated clotting time between 300 and 350 s. Next, electroanatomical maps of PVs and the left atrium were reconstructed using a multi-electrode mapping catheter (Pentaray; Biosense). Subsequently, a 56-porous irrigated-tip contact force-sensing catheter (Thermocool SmartTouch SF; Biosense) was introduced into the left atrium. This catheter was used for the localization and ablation of the antrum ostium of the PVs, with the Swartz sheath serving as the conduit for its placement. Meanwhile, the mapping catheter remained positioned within the PVs to monitor the variation of potentials during the ablation process. In addition, an electrophysiological examination was carried out in the SVC. If potential was detected, SVCI was performed at the level of the right pulmonary vein isolation (PVI) circle roofline after locating the phrenic nerve by high-output pacing (10 mA) on the lateral wall (Figure 1).
The radiofrequency energy was delivered by an ablation index-guided high-power ablation strategy of 50 W (irrigation flow 15 mL/min). For PVI, the target ablation index value was set at 450 to 550, with 10 to 15 g of contact force for anterior walls, and at 350 to 450, with 5 to 10 g of contact force for posterior walls. Ablation index values of 300 to 350, with 5 to 10 g of contact force, were applied on the lateral wall of the SVC, while 350 to 400 with similar contact force were applied on the non-lateral wall. Predefined VisiTag (Biosense) settings (the lesion-tag size 2 mm, interlesion distance 4 mm, minimum time 3 s) were used to automatically display radiofrequency applications.
WORKFLOW OF THE CONVENTIONAL GROUP:
The procedure was performed under conscious sedation. A multipolar catheter (Biosense) was placed into the coronary sinus via the jugular or subclavian vein. A single transseptal puncture was performed using a Swartz sheath under X-ray guidance. Heparin was given to maintain activated clotting time of 300 to 350 s. The mapping of left atrium and PVs was done using a Pentaray catheter and PVI was performed using a 6 porous irrigated-tip contact force sensing catheter (ThermoCool SmartTouch; Biosense) through the Swartz sheath. Additional SVCI was performed only when SVC-triggered AF was observed during the procedure. The delivery power was set to 35 W under the predefined VisiTag settings (with a lesion-tag size of 2 mm, interlesion distance of 6 mm, minimum time of 3 s). The specific ablation index and contact force values for each lesion chosen were left to the discretion of the catheter operator.
The immediate endpoint of our study was the absence or dissociation of potential from PVs and SVC and bilateral conduction block. In cases in which these endpoints were not met, we promptly conducted touch-up ablations at the earliest activation site. No liner ablation was performed unless a related atrial arrhythmia occurred. After a waiting period of more than 20 min, repeat electrophysiological examinations were performed using the Pentaray catheter during sinus rhythm, and pacing was performed along lesions to identify acute reconnections of PVs and SVC. If reconnections were identified, touch-up ablations were further performed. The ablation endpoint was bidirectional block and absence or dissociation of potential from PVs or SVC until the end of the procedure.
Acute effectiveness outcomes were defined as the first-pass isolation of bilateral PVI circles and the absence of acute PVRs. We also recorded the total procedural time, total operation time, and fluoroscopy time as relevant metrics. To evaluate the continuity and efficacy of PVI circles, case data about the performance of PVIs from CARTO was subjected to an artificial intelligence analytics platform. In this platform, the ablation index, contact force, delivery time, and location of every lesion tag were recorded and analyzed. We assessed the interlesion tag distances to identify fractured points, stacked points, and gaps. Fractured points were defined as lesion tags within a distance of ≤3 mm from any 4 points. Stacked points were characterized as lesion tags numbering ≥6 within a 6 mm radius with 1 tag as the center. Gaps were defined as interlesion distances ≥6 mm. We also examined whether the radiofrequency index reached the target ablation index value and whether contact force met the force over time (FOT) criteria, represented through graphical illustrations. FOT was specifically defined as the duration during which practical contact force reached the target contact force and accounted for more than 40% of the delivery time. Furthermore, we integrated the operation time for performing bilateral PVI circles into our assessment to evaluate procedural efficiency. To assess the quality of PVI circles, we considered the parameters of fractured points, stacked points, and gaps, as well as the proportion of radiofrequency index reaching the target value and contact force meeting the FOT criteria.
FOLLOW-UP:
Patients were scheduled for follow-up at 1 and 3 months and every 3 months thereafter, or whenever there were signs of arrhythmia. A 12-lead electrocardiogram and 24-h Holter monitoring were conducted at each visit. After a 3-month blanking period, any symptomatic or asymptomatic atrial arrhythmia lasting longer than 30 s was considered as an AF recurrence.
STATISTICAL ANALYSIS:
All statistical analyses were conducted using the RStudio software (RStudio, Boston, MA, USA) and SPSS v25.0 software (IBM Corp, Armonk, NY, USA). Propensity score matching was used to minimize potential confounding bias when selecting cases. Continuous variables are presented as the mean±the standard deviation (SD) for normally distributed variables and were compared using the
Results
PATIENT CHARACTERISTICS:
The patient characteristics of each group are shown in Table 1. The baseline characteristics were not significantly different between the 2 groups.
PROCEDURAL OUTCOMES:
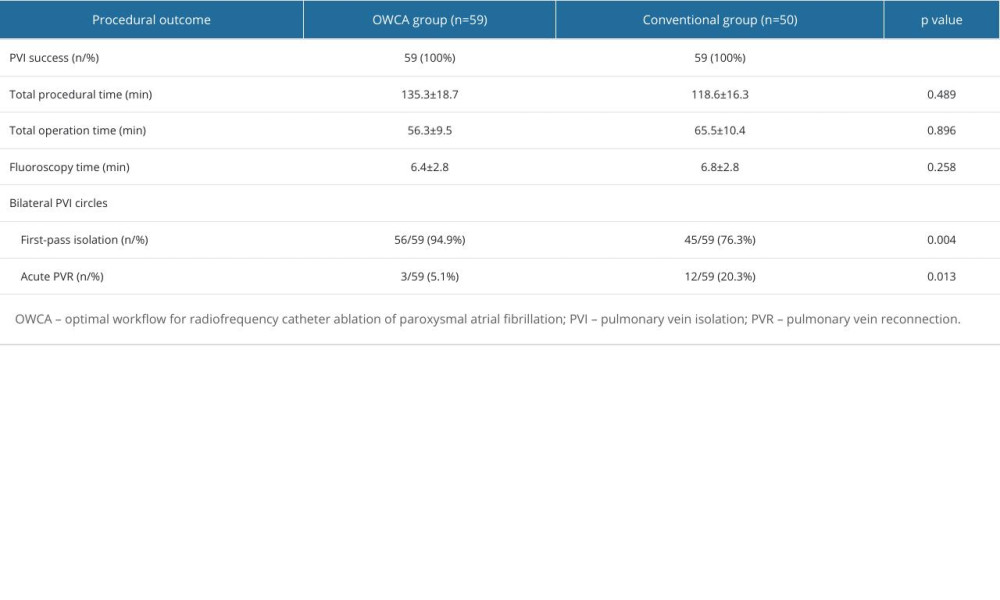
As shown in Table 2, PVI was achieved in all cases. There were no statistically significant differences between the 2 groups in terms of the total procedural time, total operation time, and fluoroscopic time. In contrast, the incidence of first-pass isolation of bilateral PVI circles was higher (94.9% vs 73.6%, P=0.009), while the incidence of the acute PVR was lower (5.1% vs 20.3%, P=0.027) in the OWCA group than in the conventional group.
ARTIFICIAL INTELLIGENCE ANALYTICS PLATFORM OUTCOMES:
A representative case illustrating the outcomes of PVI circles evaluated by artificial intelligence analytics platform is shown in Figure 2. The outcomes of the artificial intelligence analytics platform between the 2 groups are summarized in Figure 3. There were significantly fewer fractured points (6.8% vs 37.3%, P<0.001), and stacked points (3.4% vs 23.7%, P=0.003) in the OWCA group than in the conventional group. There was no gap in the OWCA group, while 3 gaps were observed in the conventional group. Additionally, the OWCA group demonstrated a higher proportion of radiofrequency index reaching the target value (96.6% vs 76.3%, P=0.003) and contact force meeting FOT (91.5% vs 52.5%, P<0.001) than did the conventional group. Furthermore, the OWCA group achieved significantly shorter operation times (42.4±6.1 vs 57.2±8.8, P=0.017) during bilateral PVI circle procedures.
FOLLOW-UP:
All patients underwent a minimum of 12 months of follow-up after the initial procedure. In the OWCA group, 2 patients experienced AF recurrence, detected by Holter monitoring. Notably, 1 patient had pulmonary vein reconnection, which was observed during a secondary ablation procedure. In the Conventional group, 9 patients experienced recurrences, including 1 case of atrial tachycardia and 8 cases of AF. A secondary ablation was performed in 4 patients, and all exhibited reconnections of PVI. Kaplan-Meier curves analysis revealed that the atrial arrhythmia-free survival rate was higher in the OWCA group than in the conventional group (Figure 4, log-rank test, P=0.015).
COMPLICATIONS:
Both cohorts experienced a low rate of complications, with no significant difference (5.1% vs 1.7%,
Discussion
STUDY LIMITATIONS:
This study had several limitations, including the small sample size from a single center. Also, patients following the optimized workflow were compared to a matched control group in a non-randomized manner. To establish the safety and effectiveness of OWCA, larger-scale multi-center studies with prospective randomized designs are warranted. Moreover, the follow-up duration was limited to 12 months, thus long-term efficacy was not thoroughly evaluated. Despite recording a 24-h Holter electrocardiogram at every visit, the use of discontinuous monitoring can underestimate the recurrence of atrial arrhythmia.
Conclusions
OWCA, involving general anesthesia, ablation index-guided high-power ablation strategy, visualized steerable sheaths, intracardiac echocardiography, and additional SCVI, had demonstrated effectiveness in producing higher quality PVI circles and improving acute and 12-month clinical outcomes.
Figures
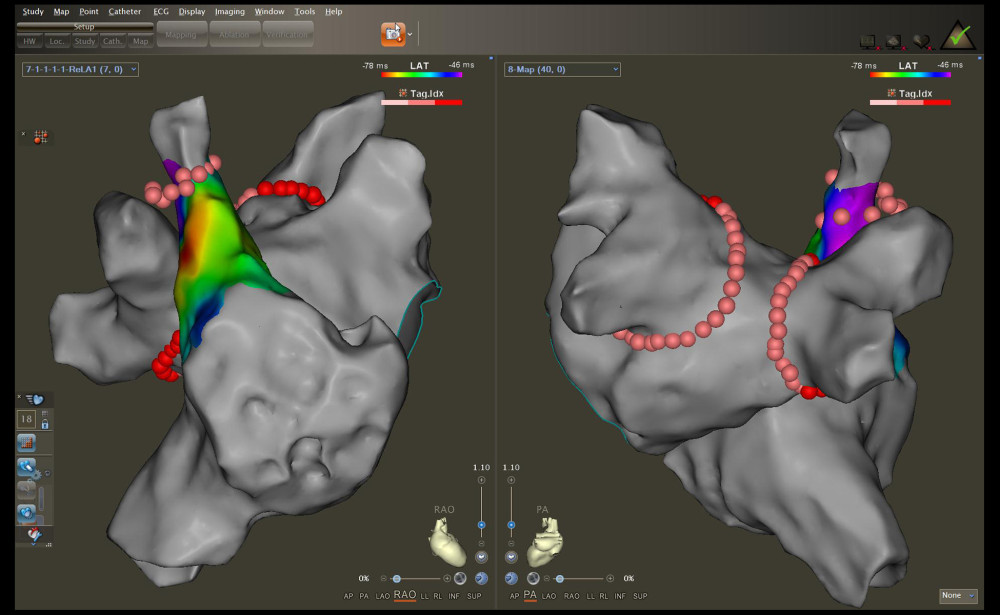 Figure 1. Radiofrequency (RF) applications to the pulmonary vein antrum and superior vena cava isolation (SVCI) on the reconstructed left atrium and right atrium electroanatomical maps. The left panel illustrates the right anterior oblique view, and the right panel displays the posteroanterior view.
Figure 1. Radiofrequency (RF) applications to the pulmonary vein antrum and superior vena cava isolation (SVCI) on the reconstructed left atrium and right atrium electroanatomical maps. The left panel illustrates the right anterior oblique view, and the right panel displays the posteroanterior view. 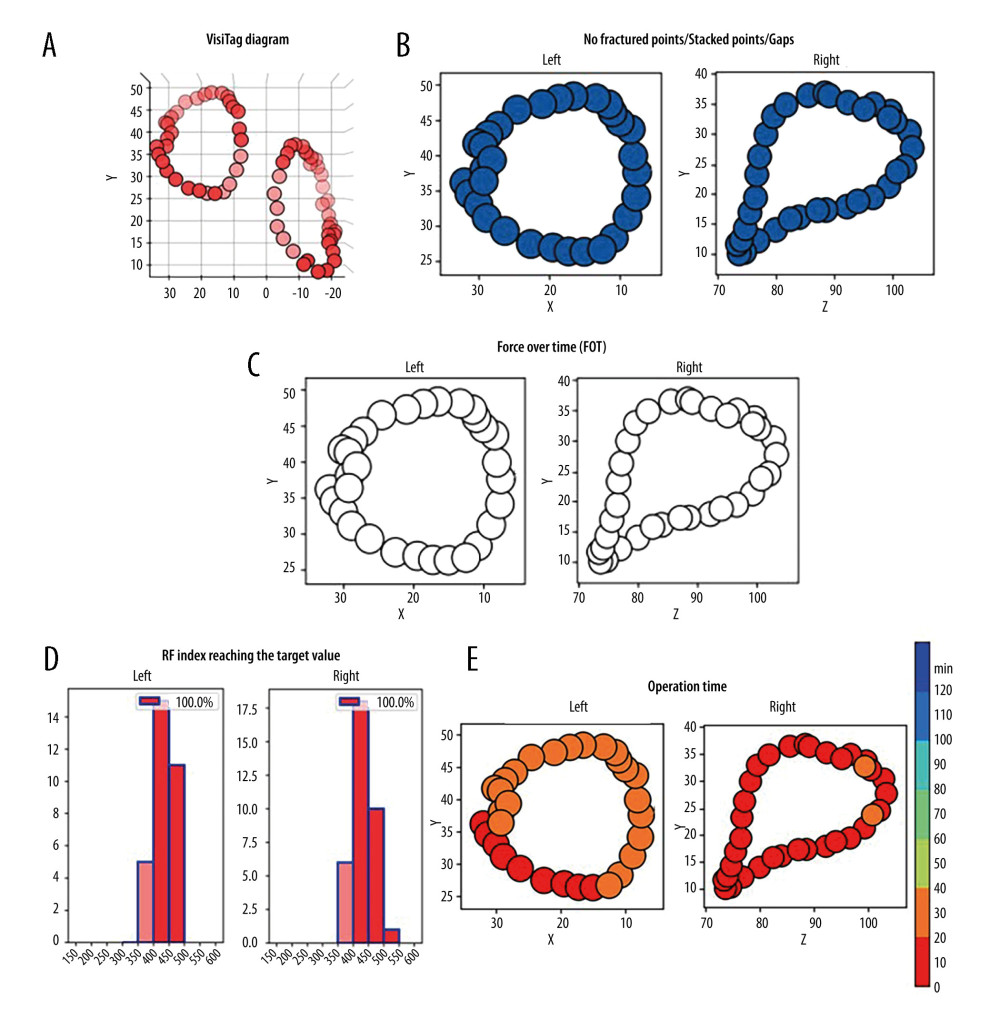 Figure 2. Representative case evaluated by an artificial intelligence analytics platform. (A) The original VisiTag diagram. (B) No fractured points (black arrow), stacked points, and gaps in bilateral pulmonary vein isolation (PVI) circles. (C) The proportion of contact force (CF) meeting force over time (FOT) was 100% in bilateral PVI circles. (D) The proportion of radiofrequency (RF) index reaching the target value was 100% in bilateral PVI circles. (E) The operation time of performing bilateral PVI circles was less than 40 min.
Figure 2. Representative case evaluated by an artificial intelligence analytics platform. (A) The original VisiTag diagram. (B) No fractured points (black arrow), stacked points, and gaps in bilateral pulmonary vein isolation (PVI) circles. (C) The proportion of contact force (CF) meeting force over time (FOT) was 100% in bilateral PVI circles. (D) The proportion of radiofrequency (RF) index reaching the target value was 100% in bilateral PVI circles. (E) The operation time of performing bilateral PVI circles was less than 40 min. 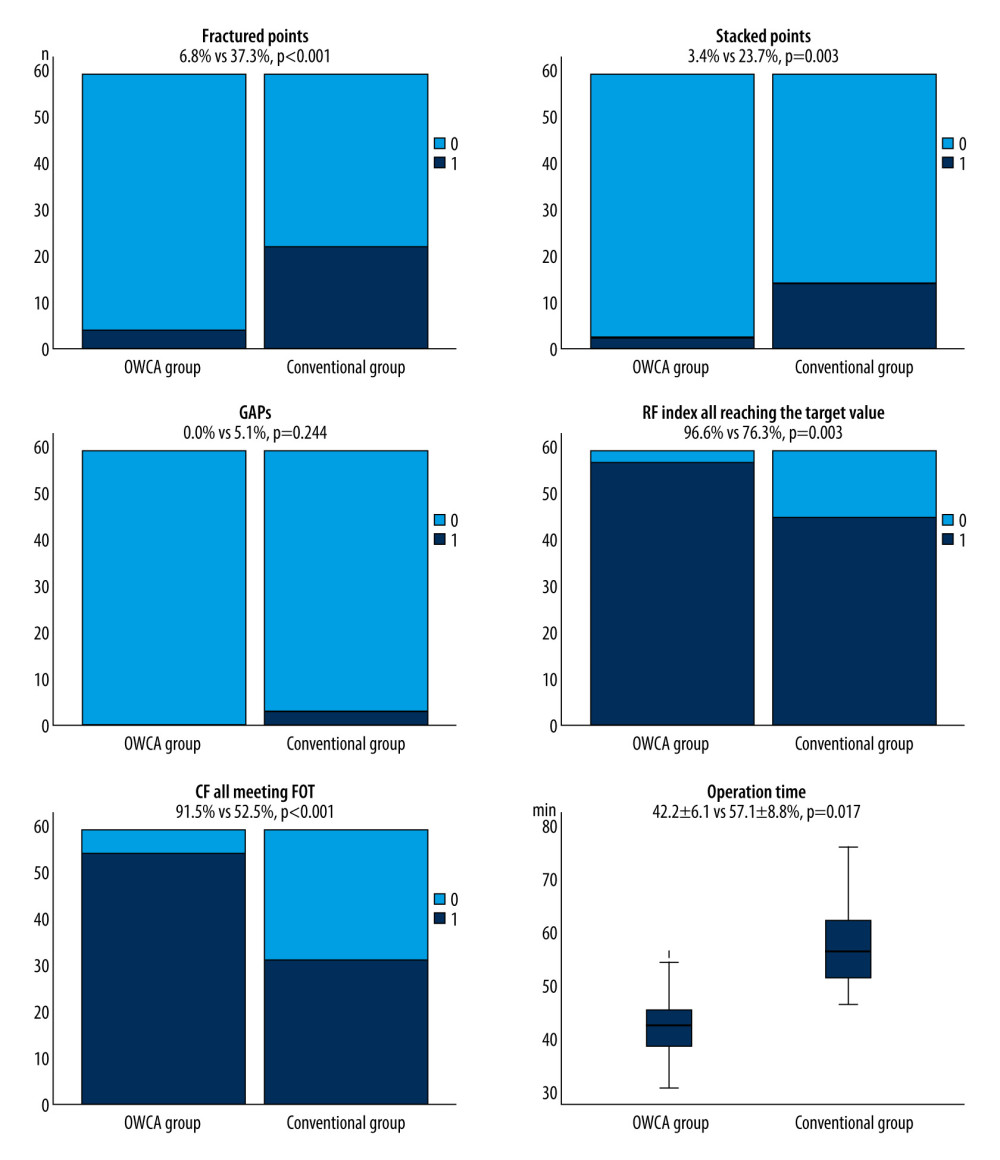 Figure 3. Comparison between the optimal workflow for radiofrequency catheter ablation of paroxysmal atrial fibrillation (OWCA) group and the conventional group in terms of fractured points, stacked points, gaps, the proportion of radiofrequency (RF) index reaching the target value, contact force meeting force over time (FOT), and operation time.
Figure 3. Comparison between the optimal workflow for radiofrequency catheter ablation of paroxysmal atrial fibrillation (OWCA) group and the conventional group in terms of fractured points, stacked points, gaps, the proportion of radiofrequency (RF) index reaching the target value, contact force meeting force over time (FOT), and operation time. 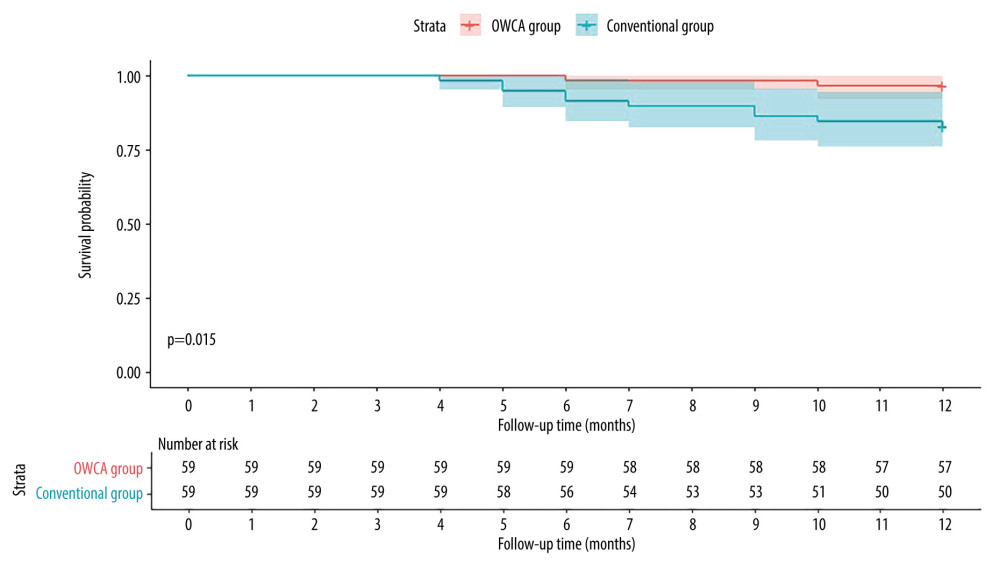 Figure 4. Kaplan-Meier curves comparing the occurrence of atrial arrhythmia after the initial ablation procedure between the optimal workflow for radiofrequency catheter ablation of paroxysmal atrial fibrillation (OWCA) group (red line) and the conventional group (blue line).
Figure 4. Kaplan-Meier curves comparing the occurrence of atrial arrhythmia after the initial ablation procedure between the optimal workflow for radiofrequency catheter ablation of paroxysmal atrial fibrillation (OWCA) group (red line) and the conventional group (blue line). References
1. Pappone C, Augello G, Sala S, A randomized trial of circumferential pulmonary vein ablation versus antiarrhythmic drug therapy in paroxysmal atrial fibrillation: the APAF Study: J Am Coll Cardiol, 2006; 48; 2340-47
2. Di Biase L, Mohanty P, Mohanty S, Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: Results from the AATAC multicenter randomized trial: Circulation, 2016; 133; 1637-44
3. Packer DL, Mark DB, Robb RA, Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: The CABANA randomized clinical trial: JAMA, 2019; 321; 1261-74
4. Hindricks G, Potpara T, Dagres N, 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): Eur Heart J, 2021; 42; 373-498
5. Ouyang F, Tilz R, Chun J, Long-term results of catheter ablation in paroxysmal atrial fibrillation: Circulation, 2010; 122; 2368-77
6. Takigawa M, Takahashi A, Kuwahara T, Impact of non-pulmonary vein foci on the outcome of the second session of catheter ablation for paroxysmal atrial fibrillation: J Cardiovasc Electrophysiol, 2015; 26; 739-46
7. Shah S, Barakat AF, Saliba WI, Recurrent atrial fibrillation after initial long-term ablation success: electrophysiological findings and outcomes of repeat ablation procedures: Circ Arrhythm Electrophysiol, 2018; 11; e005785
8. Marijon E, Fazaa S, Narayanan K, Real-time contact force sensing for pulmonary vein isolation in the setting of paroxysmal atrial fibrillation: procedural and 1-year results: J Cardiovasc Electrophysiol, 2014; 25; 130-37
9. Afzal MR, Chatta J, Samanta A, Use of contact force sensing technology during radiofrequency ablation reduces recurrence of atrial fibrillation: A systematic review and meta-analysis: Heart Rhythm, 2015; 12; 1990-96
10. Zanchi S, Chen S, Bordignon S, Ablation Index-guided high-power (50 W) short-duration for left atrial anterior and roofline ablation: Feasibility, procedural data, and lesion analysis (ablation index High-Power Linear Ablation): J Cardiovasc Electrophysiol, 2021; 32; 984-93
11. Otsuka N, Okumura Y, Kuorkawa S, Actual tissue temperature during ablation index-guided high-power short-duration ablation versus standard ablation: Implications in terms of the efficacy and safety of atrial fibrillation ablation: J Cardiovasc Electrophysiol, 2022; 33; 55-63
12. Deyell MW, Wen G, Laksman Z, The impact of steerable sheaths on unblinded contact force during catheter ablation for atrial fibrillation: J Interv Card Electrophysiol, 2020; 57; 417-24
13. Khalaph M, Sommer P, Lucas P, First clinical experience using a visualized sheath for atrial fibrillation ablation: Pacing Clin Electrophysiol, 2022; 45; 922-29
14. Hayashi K, Okumura K, Okamatsu H, Real-time visualization of the esophagus and left atrial posterior wall by intra-left atrial echocardiography: J Interv Card Electrophysiol, 2022; 63; 629-37
15. Pimentel RC, Rahai N, Maccioni S, Differences in outcomes among patients with atrial fibrillation undergoing catheter ablation with versus without intracardiac echocardiography: J Cardiovasc Electrophysiol, 2022; 33; 2015-47
16. Ejima K, Kato K, Iwanami Y, Impact of an empiric isolation of the superior vena cava in addition to circumferential pulmonary vein isolation on the outcome of paroxysmal atrial fibrillation ablation: Am J Cardiol, 2015; 116; 1711-16
17. Lévy S, Steinbeck G, Santini L, Management of atrial fibrillation: Two decades of progress – a scientific statement from the European Cardiac Arrhythmia Society: J Interv Card Electrophysiol, 2022; 65; 287-326
18. Di Biase L, Conti S, Mohanty P, General anesthesia reduces the prevalence of pulmonary vein reconnection during repeat ablation when compared with conscious sedation: Results from a randomized study: Heart Rhythm, 2011; 8; 368-72
19. Garcia R, Waldmann V, Vanduynhoven P, Worldwide sedation strategies for atrial fibrillation ablation: current status and evolution over the last decade: Europace, 2021; 23; 2039-45
20. Piorkowski C, Eitel C, Rolf S, Steerable versus nonsteerable sheath technology in atrial fibrillation ablation: A prospective, randomized study: Circ Arrhythm Electrophysiol, 2011; 4; 157-65
21. Ullah W, Hunter RJ, McLean A, Impact of steerable sheaths on contact forces and reconnection sites in ablation for persistent atrial fibrillation: J Cardiovasc Electrophysiol, 2015; 26; 266-73
22. Fitzpatrick N, Mittal A, Galvin J, The impact of steerable sheath visualization during catheter ablation for atrial fibrillation: Europace, 2023; 25; 1345-51
23. Rajendra A, Hunter TD, Morales GX, Steerable sheath visualizable under 3D electroanatomical mapping facilitates paroxysmal atrial fibrillation ablation with minimal fluoroscopy: J Interv Card Electrophysiol, 2023; 66; 381-88
24. Cui L, Chu Y, Han Y, Dong S, Comparison of higher-power and conventional power ablation of atrial fibrillation using contact force-sensing catheters: A systematic review and meta-analysis: J Interv Card Electrophysiol, 2021; 62; 1-7
25. Hussein A, Das M, Riva S, Use of ablation index-guided ablation results in high rates of durable pulmonary vein isolation and freedom from arrhythmia in persistent atrial fibrillation patients: The PRAISE study results: Circ Arrhythm Electrophysiol, 2018; 11; e006576
26. Cui L, Cui S, Dong S, Ablation index-guided high-power ablation for superior vena cava isolation in patients with atrial fibrillation: Front Cardiovasc Med, 2022; 9; 1033297
27. Vrachatis DA, Papathanasiou KA, Kossyvakis C, Efficacy, safety and feasibility of superior vena cava isolation in patients undergoing atrial fibrillation catheter ablation: An up-to-date review: Biomedicines, 2023; 11; 1022
28. El Haddad M, Taghji P, Phlips T, Determinants of acute and late pulmonary vein reconnection in contact force-guided pulmonary vein isolation: Identifying the weakest link in the ablation chain: Circ Arrhythm Electrophysiol, 2017; 10; e004867
29. Tzou WS, Sauer WH, Radiofrequency catheter ablation of atrial fibrillation: May the force (and stability) be with you, always: JACC Clin Electrophysiol, 2020; 6; 153-56
30. Ioannou A, Papageorgiou N, Lim WY, Efficacy and safety of ablation index-guided catheter ablation for atrial fibrillation: An updated meta-analysis: Europace, 2020; 22; 1659-71
31. Sairaku A, Nakano Y, Oda N, Learning curve for ablation of atrial fibrillation in medium-volume centers: J Cardiol, 2011; 57; 263-68
Figures
 Figure 1. Radiofrequency (RF) applications to the pulmonary vein antrum and superior vena cava isolation (SVCI) on the reconstructed left atrium and right atrium electroanatomical maps. The left panel illustrates the right anterior oblique view, and the right panel displays the posteroanterior view.
Figure 1. Radiofrequency (RF) applications to the pulmonary vein antrum and superior vena cava isolation (SVCI) on the reconstructed left atrium and right atrium electroanatomical maps. The left panel illustrates the right anterior oblique view, and the right panel displays the posteroanterior view. Figure 2. Representative case evaluated by an artificial intelligence analytics platform. (A) The original VisiTag diagram. (B) No fractured points (black arrow), stacked points, and gaps in bilateral pulmonary vein isolation (PVI) circles. (C) The proportion of contact force (CF) meeting force over time (FOT) was 100% in bilateral PVI circles. (D) The proportion of radiofrequency (RF) index reaching the target value was 100% in bilateral PVI circles. (E) The operation time of performing bilateral PVI circles was less than 40 min.
Figure 2. Representative case evaluated by an artificial intelligence analytics platform. (A) The original VisiTag diagram. (B) No fractured points (black arrow), stacked points, and gaps in bilateral pulmonary vein isolation (PVI) circles. (C) The proportion of contact force (CF) meeting force over time (FOT) was 100% in bilateral PVI circles. (D) The proportion of radiofrequency (RF) index reaching the target value was 100% in bilateral PVI circles. (E) The operation time of performing bilateral PVI circles was less than 40 min. Figure 3. Comparison between the optimal workflow for radiofrequency catheter ablation of paroxysmal atrial fibrillation (OWCA) group and the conventional group in terms of fractured points, stacked points, gaps, the proportion of radiofrequency (RF) index reaching the target value, contact force meeting force over time (FOT), and operation time.
Figure 3. Comparison between the optimal workflow for radiofrequency catheter ablation of paroxysmal atrial fibrillation (OWCA) group and the conventional group in terms of fractured points, stacked points, gaps, the proportion of radiofrequency (RF) index reaching the target value, contact force meeting force over time (FOT), and operation time. Figure 4. Kaplan-Meier curves comparing the occurrence of atrial arrhythmia after the initial ablation procedure between the optimal workflow for radiofrequency catheter ablation of paroxysmal atrial fibrillation (OWCA) group (red line) and the conventional group (blue line).
Figure 4. Kaplan-Meier curves comparing the occurrence of atrial arrhythmia after the initial ablation procedure between the optimal workflow for radiofrequency catheter ablation of paroxysmal atrial fibrillation (OWCA) group (red line) and the conventional group (blue line). In Press
16 Mar 2024 : Clinical Research
Diagnostic Efficiency of ACR-TIRADS Score for Differentiating Benign and Malignant Thyroid Nodules of Vario...Med Sci Monit In Press; DOI: 10.12659/MSM.943228
08 May 2024 : Clinical Research
Effect of Individualized PEEP Guided by Driving Pressure on Diaphragm Function in Patients Undergoing Lapar...Med Sci Monit In Press; DOI: 10.12659/MSM.944022
21 Mar 2024 : Clinical Research
Impact of Serum Vitamin D, B6, and B12 and Cognitive Functions on Quality of Life in Peri- and Postmenopaus...Med Sci Monit In Press; DOI: 10.12659/MSM.943249
17 Mar 2024 : Clinical Research
Protective Role of TRPC3 Gene Polymorphism (rs10518289) in Obstructive Sleep Apnea Hypopnea Syndrome Among ...Med Sci Monit In Press; DOI: 10.12659/MSM.942667
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952