26 April 2024: Lab/In Vitro Research
The Role of Copper-Induced M2 Macrophage Polarization in Protecting Cartilage Matrix in Osteoarthritis
Dawei Geng12BCE, Rongcai Lin1BCD, Peiran Wei1ABF, Cheng Tang1CDF, Yan Xu1BCDF, Liming Wang1AF*DOI: 10.12659/MSM.943738
Med Sci Monit 2024; 30:e943738
Abstract
BACKGROUND: BACKGROUND The pathological mechanism of osteoarthritis is still unclear. The regulation of the immune microenvironment has been of growing interest in the progression and treatment of osteoarthritis. Macrophages with different phenotypes, producing different cytokines, have been linked to the mechanism of cartilage injury in osteoarthritis. Copper ions play a role in the immune response and are involved in the pathological mechanisms of osteoarthritis by affecting the metabolism of the cartilage matrix. Bioactive glass (BG) is an osteogenic material with superior biocompatibility. Here, we report on the regulatory behavior of macrophages using a copper-based composite BG material. MATERIAL AND METHODS Cu-BGC powder was prepared by sol-gel method, and scaffolds were fabricated and characterized using 3D printing. Macrophage cultures grown with Cu-BGC were examined for cell culture and proliferation. The effect of Cu-BGC on the degradation metabolism of chondrocytes, cultured in the environment of inflammatory cytokine IL-1β, was determined. In addition, the morphology of macrophages, secretion of inflammatory cytokines, and expression of surface markers were examined. RESULTS The results show that Cu-BGC promotes macrophage proliferation at a range of concentrations and increases the secretion of anti-inflammatory cytokines while inhibiting proinflammatory cytokines. At the same time, M2-type cell surface markers are definitely expressed and the morphology of macrophages is altered. In addition, Cu-BGC inhibited the degradation metabolism of chondrocytes in the inflammatory environment induced by IL-1β. CONCLUSIONS These results suggest that Cu-BGC induced macrophage polarization into an M2 type anti-inflammatory phenotype, and inhibition of immune injury response may play a role in delaying cartilage matrix damage in osteoarthritis.
MATERIAL AND METHODS:
RESULTS:
CONCLUSIONS:
Keywords: Macrophages, Immunity, Cellular, Osteoarthritis
Introduction
Osteoarthritis, which is characterized by the wear of cartilage and subchondral bone, was previously defined as a non-inflammatory degenerative disease [1]. However, evidence is mounting that metabolic responses, immune responses, and inflammation play crucial roles in the pathogenesis of osteoarthritis [2]. Particularly, immunologic injury is involved in the early stage and the progressive degeneration of osteoarthritis [3]. Macrophages are immunomodulatory cells in the immune system. Based on the different stress patterns, macrophages polarize into M1 and M2 phenotype, that is, proinflammatory phenotype (M1) and anti-inflammatory phenotype (M2) [4]. It was reported that M1 macrophages induce the hypertrophy and injury of chondrocytes by stimulating the production of matrix metalloproteinase and accelerating the progression of osteoarthritis, while M2 macrophages have a protective effect on chondrocytes [2,5,6]. Other studies show that treatments targeting macrophages relieve the pain of osteoarthritis [3]. Macrophages play a role in cartilage damage in osteoarthritis, but the specific mechanism is still unclear [1,2,5]. Therefore, research focused on macrophages has emerged as a potential direction for osteoarthritis treatment.
Copper ion is one of the components of various binding proteins in the human body [7]. It participates in cellular immunity and humoral immunity, and plays a role in pathological environments such as inflammation and infection [8]. Copper is known for its anti-inflammatory properties, and it has been documented that copper has a positive effect on the treatment of osteoarthritis and osteogenesis [9–11]. During cartilage formation or regeneration, copper-related lysine oxidase is an essential protease for collagen crosslinking in cartilage matrix [12,13].
Osteoarthritis has various pathological manifestations, including synovitis, wear of cartilage, injury of subchondral bone, formation of osteophytes, and joint deformity [14,15]. Medications for osteoarthritis are symptomatic treatment, while end-stage osteoarthritis is treated by arthroplasty [14]. There is insufficient evidence for development of therapies to delay the progression of osteoarthritis. Thus, treating early osteoarthritis or delaying its progression is critical, and at the same time, the regulation of the immune microenvironment in osteoarthritis has received increasing attention. Bioactive glass is a mature silica-calcium mineral material with good bone conduction properties, which is widely used in research of materials for treatment of osteoarthritis [16]. Thus, the incorporation of copper into bioactive glasses may have the ability to modulate macrophages polarization towards an anti-inflammatory phenotype, thereby promoting cartilage regeneration and delaying the progression of osteoarthritis. Here, we investigated the effect of copper-containing bioactive glasses on macrophages in the context of osteoarthritis.
Material and Methods
CU-BGC POWDERS AND SCAFFOLDS PREPARATION AND CHARACTERIZATION:
Cu-BGC were produced by sol-gel method based on our literature review [17]. First, we measured out 157 mL TEOS and 25 mL HNO3, and added it into 100 mL ultrapure water, and stirred continuously for half an hour to get the mixture, then 12.08 g Cu(NO3)2 (64 mmol) and 47.23 g Ca(NO3)2 (287.8 mmol) and 8.53 mL (C2H5O)3PO were added into the aforesaid mixture by stirring 5 hours to obtain the wet gel. After drying the wet gel at 120°C for 24 hours and grinding, the powder was sifted. The BGC powder was prepared in a similar manner.
The powders were mixed with F-127 and sodium alginate to obtain the printing ink, which was fabricated using 3D printing. Scaffolding was sintered at 800°C (2°C min−1) to obtain the final product. The morphology of scaffolds was characterized using SEM (JEOL; Tokyo, Japan).
CELL CULTURE OF MACROPHAGES:
The macrophages (RAW264.7) were acquired from Cobioer Biosciences (China). Raw264.7 was incubated with the high-glucose DMEM medium (Life Technologies, America) containing 10% fetal bovine serum and 1% penicillin/streptomycin at 37°C in a 5% CO2 incubator, and the medium was changed once a day.
THE PROLIFERATION OF MACROPHAGES INCUBATED WITH CU-BGC:
The proliferation of macrophages cultured with different concentrations of Cu-BGC extract was detected by cell counting kit-8 (CCK-8, Sigma, USA). The original concentration of Cu-BGC solution was 200 mg/mL, and the solution was diluted in equal volumes to different concentration gradients, with the lowest concentration being 0.781 mg/mL. The RAW264.7 cells were incubated for 1, 2, or 3 days in 96-well plates at a density of 10 000 cells/well. The cells were then co-cultured with a 10% CCK-8 kit in an incubator for 90 minutes. The absorbance of the dielectric was read out at 450 nm wavelength by a Spectra Fluor Plus microplate reader.
Extracts from BGC and Cu-BGC were obtained based on the following protocol. First, the powder was de-scented in high-glucose DMEM at a ratio of 200 mg/mL and oscillated for 24 h. Then, the mixtures were centrifuged and the supernatant liquid was filtrated through a 0.22-μM filter. Finally, the extracts were equally diluted to different concentrations.
THE POLARIZATION PHENOTYPE OF MACROPHAGES CULTURED WITH CU-BGC:
RT-qPCR was used to detect expression of inflammatory factors and surface markers in macrophages grown in 6-well plates for 3 days with different concentration gradients of BGC and Cu-BGC extracts. Then, RNA was obtained by treating the macrophages with the TRIzol kit (TOYOBO, Japan). After detecting RNA concentrations, cDNA was prepared using All-In-One RT MasterMix (TOYOBO, Japan). The RT-qPCR process was completed in Light Cycler 480 II (Roche, CHE). Finally, we calculated the expression of target genes using 2−ΔΔt. The sequences of all primers for the target genes are given in Table 1, and the TRIzol kit information and the concentration of RNA are given in Tables 2 and 3.
THE CYTOSKELETAL MORPHOLOGY OF MACROPHAGES CULTURED WITH CU-BGC:
After 3 days of BGC and Cu-BGC culturing, the macrophages were fixed with glutaraldehyde and then incubated with 1% BSA. The cytoskeleton was dyed with FITC (1 mg/mL, Sigma Aldrich, USA), and the nucleus was dyed with DAPI (Sigma Aldrich, USA). Lastly, the fluorescence images were observed by confocal microscope (CLSM, Leica TCS SP2).
THE DEGRADATION METABOLISM OF CHONDROCYTES CULTURED WITH CU-BGC:
The chondrocytes were incubated with DMEM to 80% density. Then, the chondrocytes were co-cultured with 10 ng/ml IL-1β and different concentrations of Cu-BGC. The expression of genes involved in the degradation metabolism of chondroitin was detected using the PCR method described above. The expression of genes related to the degradation metabolism of chondrocytes incubated with Cu2+ ions was examined in the same way. The sequences of all primers for the target genes are given in Table 4.
STATISTICAL ANALYSIS:
All quantitative data are expressed as means±standard deviation. A one-way ANOVA test was used to analyze the comparison between the groups. * P<0.05, *P<0.01, ** P<0.001.
Results
CHARACTERIZATION OF CU-BGC:
Cu-BGC appeared blue, indicating that it had the characteristic color of copper ions, and scanning electron microscope showed that Cu-BGC had a slight amount of amorphous phase crystallization (Figure 1). The crystal phase of BGC was SiO2, after incorporation of copper in the BGC there was a small quantity of amorphous phase in Cu-BGC, and the new phases formation was Ca20Cu(PO4)14.
THE IMPACT OF CU-BGC ON PROLIFERATION OF MACROPHAGES:
The proliferation of macrophages bred with Cu-BGC was promoted at 0.781–3.125 mg/mL (Figure 2A). Macrophages were incubated with different concentrations of Cu ions, depending on the ionic concentration of the Cu-BGC extract by ICP. The results showed that Cu ions had no obvious toxicity to macrophages, while the concentrations of Cu were less than 16 ppm. There was a significant effect on the proliferation of macrophages at concentrations below 2.0 ppm, in comparison with blank control groups (Figure 2B).
CU-BGC EXTRACTS LEADING MACROPHAGES POLARIZATION TO ANTI-INFLAMMATION PHENOTYPE:
To assess the ability of Cu-BGC to polarize macrophages, surface markers and cytokine expression were investigated. IL-1beta and IL-6, which are typical proinflammatory factors, were inhibited in macrophages grown with Cu-BGC at concentrations of 0.78–25 mg/mL (Figure 3A, 3B). Meanwhile, IL-4, an anti-inflammatory cytokine, was improved by comparison to BGC and CTR groups within the same concentration range (Figure 3C). Macrophage surface markers were examined. CD86 and CCR7 are representative surface markers of M1 macrophages, and Arg-1 is a typical surface marker of M2 macrophages. After the macrophages were cultured with Cu-BGC for 1 and 3 days, the expressions of CD86 and CCR7 were downregulated at a concentration range of 0.78–25 mg/mL, while the expression of Arg-1 was upregulated (Figure 3D–3F).
THE POLARIZATION OF CU ON MACROPHAGES:
The cell surface markers and cytokines of macrophages were analyzed by RT-qPCR. The expressions of proinflammatory cytokines, including IL-1 beta and IL-6, were suppressed in macrophages incubated with Cu ions within a certain concentration range in comparison with the control group at day 3 (Figure 4A, 4B). The expression levels of CD86 and CCR7, which are M1 macrophages surface markers, were downregulated at concentrations of 0.5–16 ppm (Figure 4D–4F). We also examined the anti-inflammatory factor IL-4, showing that it was promoted after culturing with Cu ions compared to the control group (Figure 4C). Arg-1 expression in macrophages co-cultured with a concentration range of Cu ions (0.5–16 ppm) was elevated (Figure 4F). These results suggest that copper can induce macrophage polarization into the M2 phenotype.
THE CELL MORPHOLOGY OF MACROPHAGE POLARIZATION:
The effect of macrophage polarization on cell morphology was observed using cytoskeletal staining. It was noted that macrophages were circular in shape in both the control and BGC groups, with the exception of a few cells with some morphological changes. The macrophages cultured with Cu-BGC extracts were elongated at different concentrations (Figure 5A–5F). As the concentration of Cu-BGC extracts increased, more and more macrophages became elongated, especially at the concentration of 25 mg/mL (Figure 5D2–5D6, 5F2–5F6). To confirm the role of Cu2+ in the effect of Cu-BGC on cell morphological changes in macrophages, macrophages were cultured with a homologous concentration of Cu2+ solution. Subsequently, the cell shape of macrophages was elongated at a certain concentration of Cu ions and the macrophages was elongated at 16 ppm (Figure 6A–6C). This suggests that Cu ions induce macrophage polarization into the M2 phenotype and also affect macrophage morphology.
CU-BGC INHIBITED THE DEGRADATION METABOLISM OF CHONDROCYTES:
The degradation metabolism of chondrocytes was detected using RT-qPCR. Compared with the control group and the BGC group, the expressions of MMP3, MMP13 and Adamts5 (Figure 7A–7C) were downregulated in chondrocytes cultured with Cu-BGC at concentrations of 0.781–25 mg/ml. These genes, including MMP3, MMP13, and Adamts5 (Figure 7D–7F), had restrained expression when incubated with Cu2+ ions at concentrations of 0.5–16 ppm.
Discussion
The pathogenesis of osteoarthritis is not fully understood, especially the role and mechanism of the immune microenvironment and immune response in osteoarthritis, which are still under study. Recent research results show that the development mechanism of macrophages in osteoarthritis mainly involves degradation metabolism [1,18–20]. Copper is an essential element in immune response, and copper deficiency significantly affects antibacterial activity [8]. The present study shows that Cu-BGC induces proliferation and polarization of macrophages into the M2 anti-inflammatory phenotype. Expressions of M2 surface markers and anti-inflammatory factors were significantly enhanced, while M1 surface markers and proinflammatory cytokines were suppressed.
Macrophages, as essential immune-regulatory cells in the immune system, possess diversity and plasticity [18,19]. The human body has different manifestations through immune response under the stimulation of inflammation. The activation of macrophages, which was influenced by the microenvironment, was polarized into 2 phenotypes (M1 and M2) [20]. The anti-inflammatory phenotype is conductive to tissue repair, while the proinflammatory phenotype induces tissue damage. M1 macrophages mainly secret proinflammatory cytokines, including IL-1β, IL-6, IL-18, and TNF-α, to promote an inflammatory response [6,21]. M2 macrophages produce anti-inflammatory cytokine to achieve immunosuppression and induce tissue repair, like as IL-4, IL-10, and TGF-beta [21,22]. In addition, macrophages with different phenotypes have different surface markers – M1 phenotype macrophages express iNOS, CCR7, and CD86, and M2 phenotype macrophages express elevated levels of Arg-1 and CD206 [21,23]. In this study, the expression of proinflammatory cytokines (IL-1 β and IL-6) and surface markers (CCR7and CD86) were inhibited in macrophages incubated with Cu-BGC in the range of 0.781-25 mg/mL. On the other hand, the expression of anti-inflammatory factor (IL-4) and surface marker (Arg-1) were significantly increased. To prove the effects of Cu2+ in Cu-BGC extracts on macrophages (RAW264.7), the cells were cultivated with corresponding concentrations of Cu ions. The results showed that anti-inflammatory cytokines were upregulated and proinflammatory cytokines were downregulated, while M2 surface markers were significantly increased and M1 surface markers were suppressed.
It was found that the morphology of macrophages differs according to the cultured media used [24,25]. The polarization of macrophages is associated with cell shape, and the morphological changes of macrophages with different phenotypes depend on the contractility of the actin cytoskeleton [24,26]. The actin cytoskeleton of M2 macrophages is slender and elongated, causing the macrophages to extend more antennae, but the actin cytoskeleton of M1 macrophages is relatively short, resulting in disc-shaped cells. In this study, we labeled actin with FITC to observe the morphology of macrophages, which were elongated and antennae-like when cultured with Cu-BGC. This result is consistent with previous reports that the M2 phenotype is elongated, while the M1 phenotype is circular or pancake-shaped. Based on the above results, Cu2+ released by Cu-BGC extract induces polarization of macrophages into the M2 phenotype, promoting anti-inflammatory effects in the low concentration range.
The role of macrophages in osteoarthritis has been gradually defined over time. The typical pathological manifestation of osteoarthritis is the wear and degeneration of cartilage [27]. Studies have shown that macrophages participate in the pathological process of osteoarthritis by secreting cytokines or mediators, M1 macrophages upregulate inflammatory factors and matrix metalloproteinase to generate arthritis and induce joint degeneration, and M2 macrophages induce some regulatory cytokines to repair damaged articular cartilage [5,28,29]. In this study, matrix metalloproteinases, including MMP3 and MMP13, were downregulated by the action of Cu ions in Cu-BGC extracts on chondrocytes. Liu et al examined the phenotype of macrophages in the peripheral blood and synovial fluid of 80 patients with knee osteoarthritis, and observed that the ratio of M1/M2 in these patients was significantly higher than that in the normal control group [28]. Another study showed that the level of CD206 in anti-inflammatory macrophages in OA patients was significantly lower [30]. A damage-related molecular model was proposed: the fragments or particles of cartilage after abrasion are engulfed by macrophages; if cartilage fragments cannot be fully eliminated, they will be inflammatory mediators to act on chondrocytes and macrophages secreting proinflammatory cytokines and releasing matrix metalloproteinase, leading to a vicious cycle between chondrocytes and synovial macrophages and aggravating cartilage damage [31]. The degradation and synthesis of the cartilage matrix is disrupted and the degree of osteoarthritis is exacerbated. Macrophages induced toward the M2 phenotype release anti-inflammatory mediators to promote collagen deposition and repair cartilage damage [32]. Therefore, regenerating cartilage in osteoarthritis by activating the immune response is crucial. In the present study, Cu ions induced macrophages to polarize into M2 anti-inflammatory phenotype, downregulated proinflammatory factors, and over-expressed anti-inflammatory factors. It is reasonable to speculate that Cu-BGC can activate the immune response in osteochondral tissue and affect the immune microenvironment in joints by regulating the polarization of macrophages, thereby reducing the cartilage matrix damage and delaying the degeneration caused by osteoarthritis and promoting cartilage repair. This suggests that Cu-BGC has broad potential applications in delaying the progression of osteoarthritis.
Conclusions
We found that copper released from Cu-BGC extract promotes the proliferation of macrophages. Copper ions induced polarization of macrophages toward the M2 phenotype while inhibiting excretion of proinflammatory factors and increasing expression levels of anti-inflammatory cytokines. At the same time, copper ions inhibit the degradation metabolism of chondrocytes, thereby delaying osteoarthritis degeneration. This study suggests that Cu-BGC can modulate the immune response of macrophages, which may provide a direction for the role of macrophages in the treatment and prevention of osteoarthritis, and it may prolong the end stage of osteoarthritis by modulating the immune microenvironment in joints.
Figures
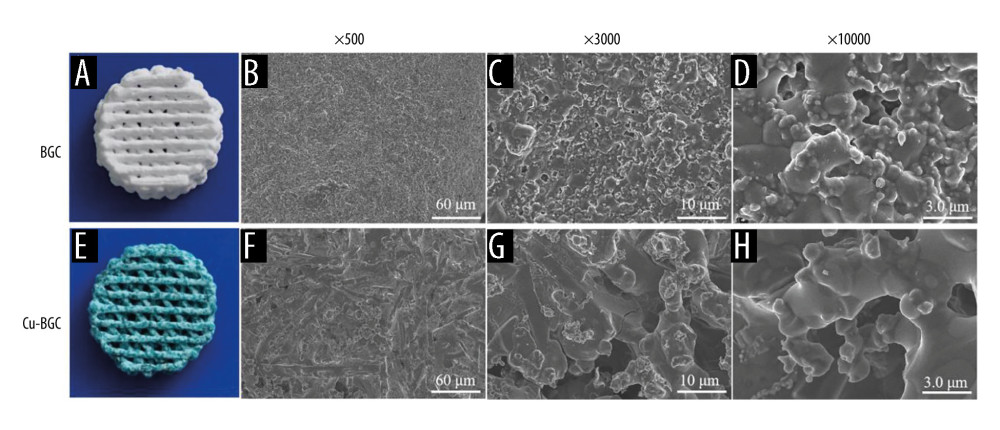 Figure 1. The structure of BGC and Cu-BGC scaffolds. The digital photographs of BGC (A) and Cu-BGC (E) scaffolds. The SEM images of BGC scaffolds (B–D) and Cu-BGC scaffolds (F–H).
Figure 1. The structure of BGC and Cu-BGC scaffolds. The digital photographs of BGC (A) and Cu-BGC (E) scaffolds. The SEM images of BGC scaffolds (B–D) and Cu-BGC scaffolds (F–H). 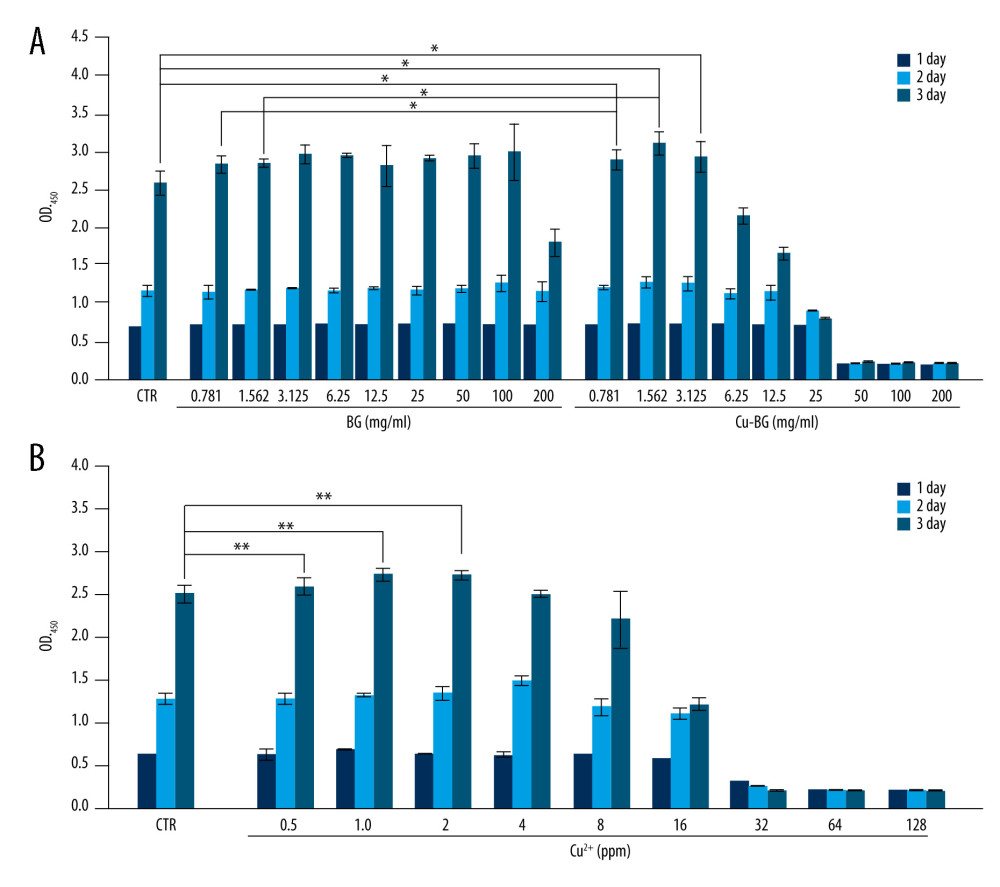 Figure 2. The proliferation of macrophages. The proliferation of macrophages cultured with different concentrations of Cu-BGC (A) and Cu2+ ions (B) enhanced within certain concentrations range (n=6, * p<0.05, ** p<0.01, *** p<0.001, respectively).
Figure 2. The proliferation of macrophages. The proliferation of macrophages cultured with different concentrations of Cu-BGC (A) and Cu2+ ions (B) enhanced within certain concentrations range (n=6, * p<0.05, ** p<0.01, *** p<0.001, respectively). 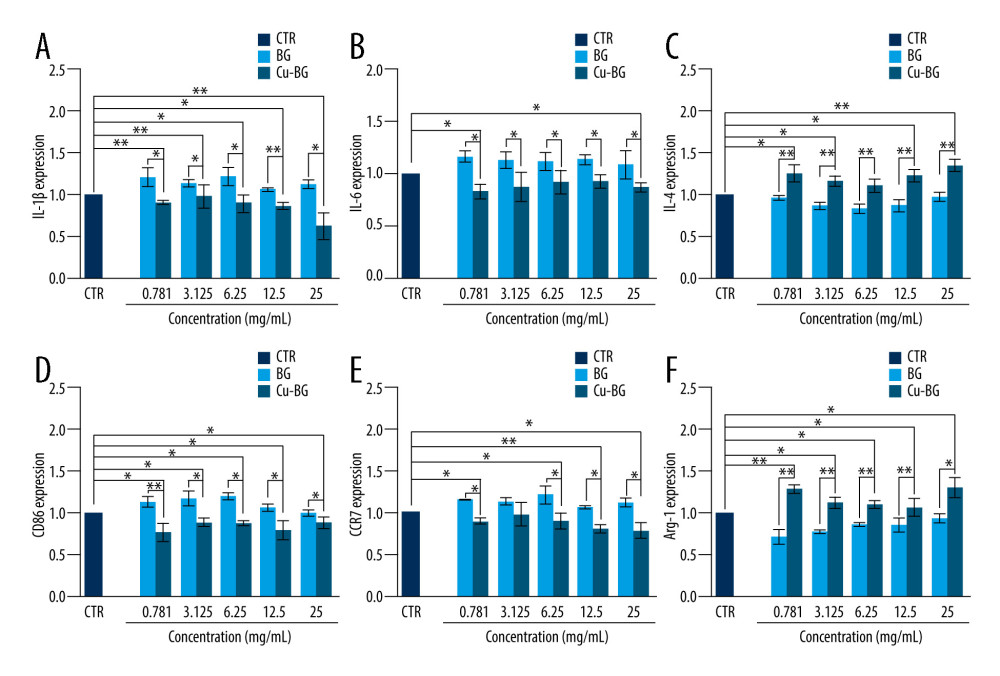 Figure 3. The expression of cytokines and surface markers in macrophages incubated with BGC and Cu-BGC. The expression of proinflammatory cytokines, IL-1β (A), IL-6 (B) and anti-inflammatory cytokine, IL-4 (C) of macrophages at day 3. The expression of M1 phenotype surface markers, CD86 (D), CCR7 (E), and M2 phenotype surface marker, Arg-1 (F) of macrophages at day 3. The relative gene amount of the CTR group was set as 1. * p<0.05, ** p<0.01, *** p<0.001, n=3.
Figure 3. The expression of cytokines and surface markers in macrophages incubated with BGC and Cu-BGC. The expression of proinflammatory cytokines, IL-1β (A), IL-6 (B) and anti-inflammatory cytokine, IL-4 (C) of macrophages at day 3. The expression of M1 phenotype surface markers, CD86 (D), CCR7 (E), and M2 phenotype surface marker, Arg-1 (F) of macrophages at day 3. The relative gene amount of the CTR group was set as 1. * p<0.05, ** p<0.01, *** p<0.001, n=3. 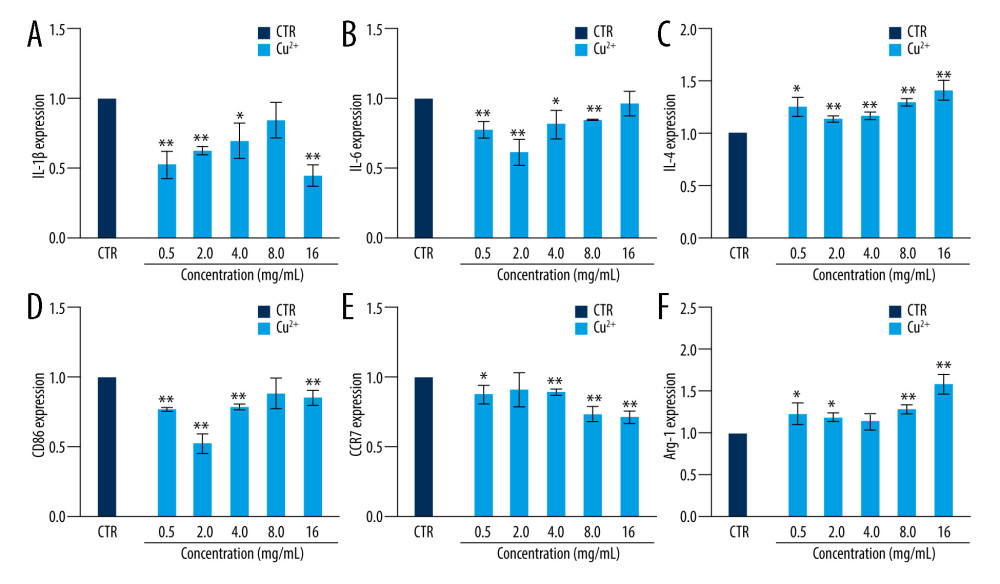 Figure 4. The effect of Cu2+ ions on the expression of cytokines and surface markers in macrophages. The expression of proinflammatory cytokines, IL-1β (A), IL-6 (B), and anti-inflammatory cytokine, IL-4 (C) of macrophages cultured with different concentrations of Cu2+ ions at day 3. The expression of M1 phenotype surface markers, CD86 (D), CCR7 (E), and M2 phenotype surface marker, Arg-1 (F) of macrophages cultured with different concentration of Cu2+ ions at day 3. The relative gene amount of CTR group was set as 1. * p<0.05, ** p<0.01, *** p<0.001, n=3.
Figure 4. The effect of Cu2+ ions on the expression of cytokines and surface markers in macrophages. The expression of proinflammatory cytokines, IL-1β (A), IL-6 (B), and anti-inflammatory cytokine, IL-4 (C) of macrophages cultured with different concentrations of Cu2+ ions at day 3. The expression of M1 phenotype surface markers, CD86 (D), CCR7 (E), and M2 phenotype surface marker, Arg-1 (F) of macrophages cultured with different concentration of Cu2+ ions at day 3. The relative gene amount of CTR group was set as 1. * p<0.05, ** p<0.01, *** p<0.001, n=3. 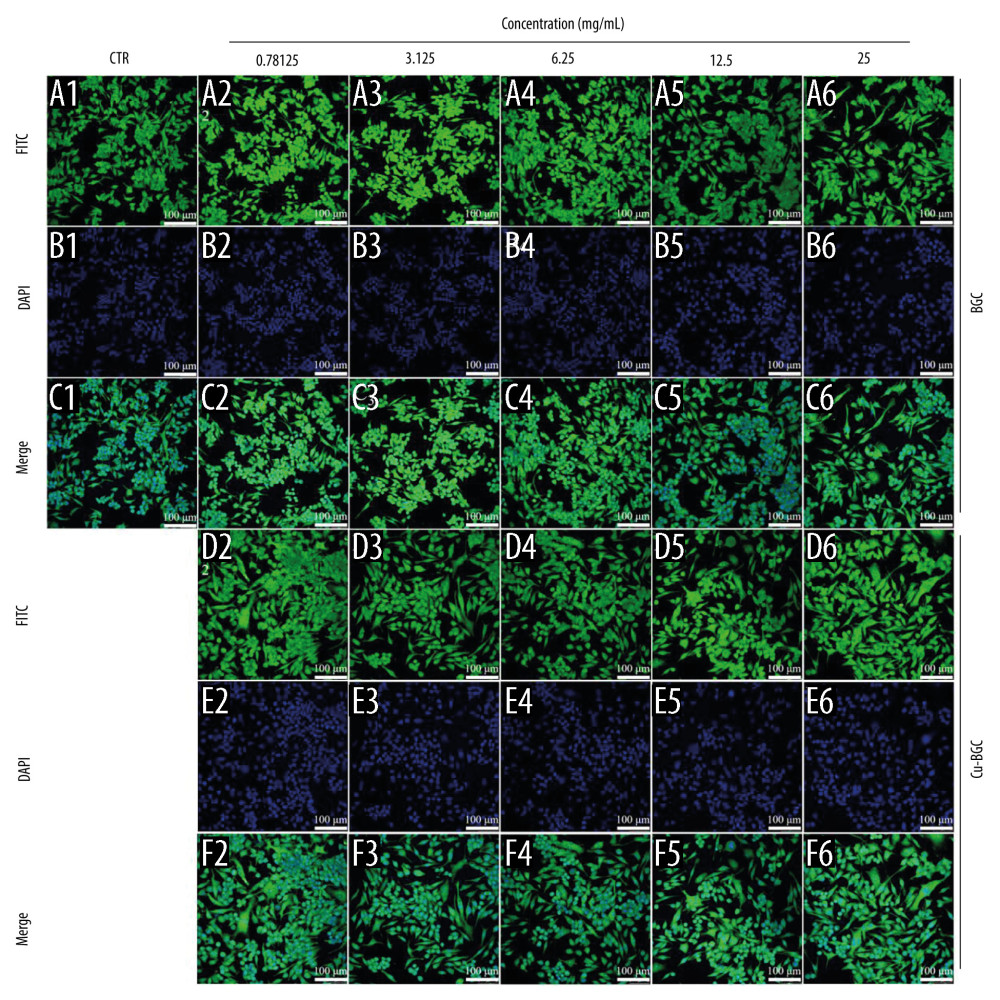 Figure 5. The morphology of macrophages incubated with BG and Cu-BG. The morphology of macrophages was examined by CLSM, including FITC (A, D), DAPI (B, E), and Merge (C, F). (scale bar=100 μm).
Figure 5. The morphology of macrophages incubated with BG and Cu-BG. The morphology of macrophages was examined by CLSM, including FITC (A, D), DAPI (B, E), and Merge (C, F). (scale bar=100 μm). 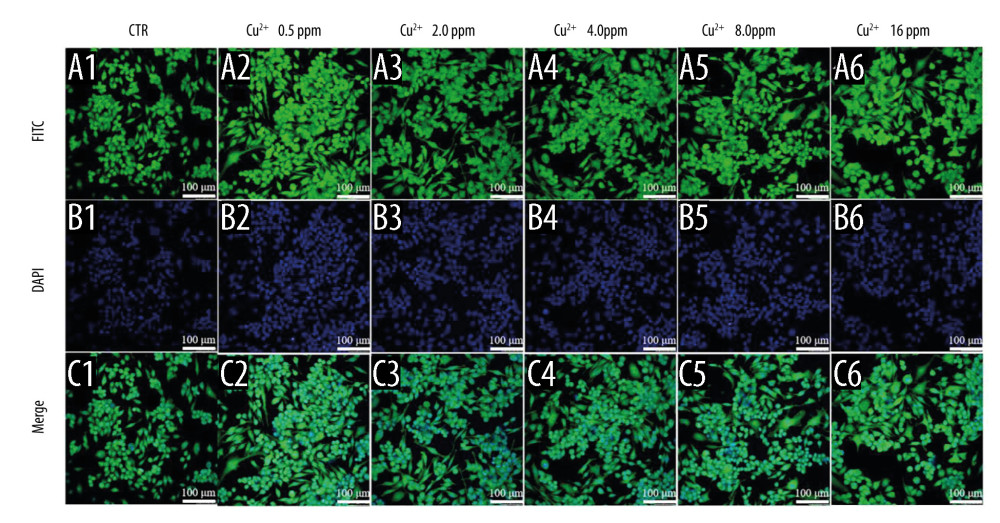 Figure 6. The morphology of macrophages incubated with different concentration of Cu2+ ions. The CLSM images characterized the morphology of macrophages, including FITC (A), DAPI (B) and Merge (C). (scale bar=100 μm)
Figure 6. The morphology of macrophages incubated with different concentration of Cu2+ ions. The CLSM images characterized the morphology of macrophages, including FITC (A), DAPI (B) and Merge (C). (scale bar=100 μm) 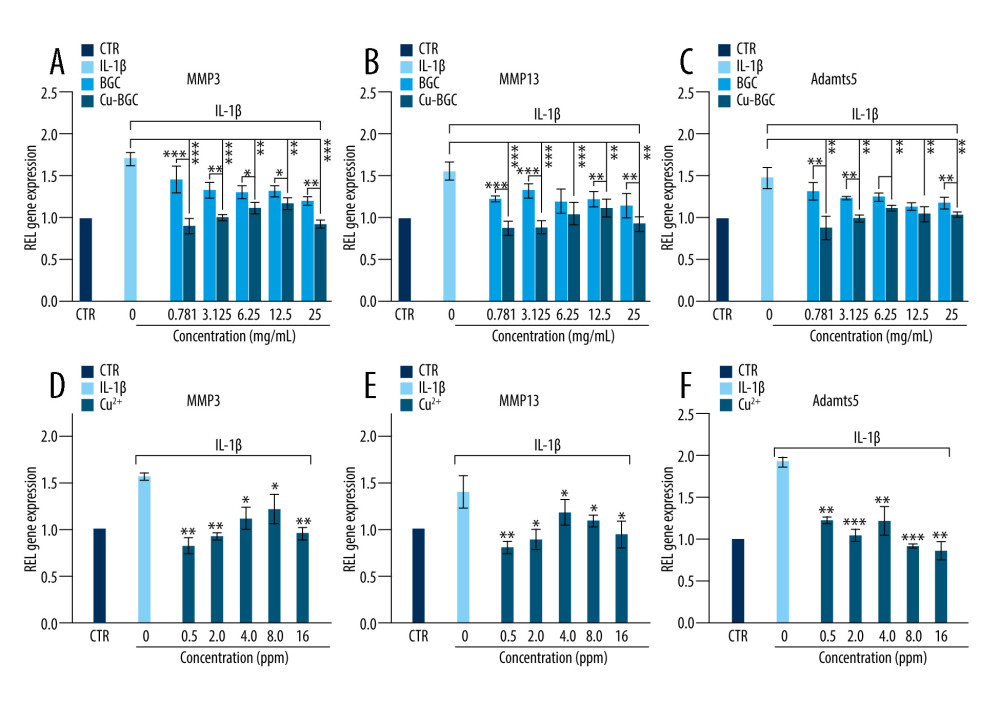 Figure 7. The degradation metabolism of chondrocytes. The expression of MMP3 (A), MMP13 (B) and Adamts5 (C) were co-cultured with 10 ng/ml IL-1β and Cu-BGC for 1 day. The expression of MMP3 (D), MMP13 (E) and Adamts5 (F) were co-cultured with 10 ng/ml IL-1β and Cu2+ ions for 1 day. The relative gene amount of CTR group was set as 1. * p<0.05, ** p<0.01, *** p<0.001, n=3.
Figure 7. The degradation metabolism of chondrocytes. The expression of MMP3 (A), MMP13 (B) and Adamts5 (C) were co-cultured with 10 ng/ml IL-1β and Cu-BGC for 1 day. The expression of MMP3 (D), MMP13 (E) and Adamts5 (F) were co-cultured with 10 ng/ml IL-1β and Cu2+ ions for 1 day. The relative gene amount of CTR group was set as 1. * p<0.05, ** p<0.01, *** p<0.001, n=3. References
1. Hunter DJ, Bierma-Zeinstra S, Osteoarthritis: Lancet, 2019; 393(10182); 1745-59
2. Kraus VB, McDaniel G, Huebner JL, Direct in vivo evidence of activated macrophages in human osteoarthritis: Osteoarthritis Cartilage, 2016; 24(9); 1613-21
3. Liang C, Wu S, Xia G, Engineered M2a macrophages for the treatment of osteoarthritis: Front Immunol, 2022; 13; 1054938
4. Locati M, Curtale G, Mantovani A, Diversity, mechanisms, and significance of macrophage plasticity: Annu Rev Pathol, 2020; 15; 123-47
5. Ma Y, Yang H, Zong X, Artificial M2 macrophages for disease-modifying osteoarthritis therapeutics: Biomaterials, 2021; 274; 120865
6. Viola A, Munari F, Sanchez-Rodriguez R, The metabolic signature of macrophage responses: Front Immunol, 2019; 10; 1462
7. Gombart AF, Pierre A, Maggini S, A review of micronutrients and the immune system-working in harmony to reduce the risk of infection: Nutrients, 2020; 12; 1
8. Cheng F, Peng G, Lu Y, Relationship between copper and immunity: The potential role of copper in tumor immunity: Front Oncol, 2022; 12; 1019153
9. Wu C, Zhou Y, Xu M, Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity: Biomaterials, 2013; 34(2); 422-33
10. Palza H, Antimicrobial polymers with metal nanoparticles: Int J Mol Sci, 2015; 16(1); 2099-116
11. Medeiros DM, Copper, iron, and selenium dietary deficiencies negatively impact skeletal integrity: A review: Exp Biol Med (Maywood), 2016; 241(12); 1316-22
12. Alshenibr W, Tashkandi MM, Alsaqer SF, Anabolic role of lysyl oxidase like-2 in cartilage of knee and temporomandibular joints with osteoarthritis: Arthritis Res Ther, 2017; 19(1); 179
13. Huang ZM, Du SH, Huang LG, Leptin promotes apoptosis and inhibits autophagy of chondrocytes through upregulating lysyl oxidase-like 3 during osteoarthritis pathogenesis: Osteoarthritis Cartilage, 2016; 24(7); 1246-53
14. Katz JN, Arant KR, Loeser RF, Diagnosis and treatment of hip and knee osteoarthritis: A review: JAMA, 2021; 325(6); 568-78
15. Abramoff B, Caldera FE, Osteoarthritis: Pathology, diagnosis, and treatment options: Med Clin North Am, 2020; 104(2); 293-311
16. Wu C, Chang J, A review of bioactive silicate ceramics: Biomed Mater, 2013; 8(3); 032001
17. Liu Y, Lin R, Ma L, Mesoporous bioactive glass for synergistic therapy of tumor and regeneration of bone tissue: Applied Materials Today, 2020; 19; 100578
18. Sica A, Mantovani A, Macrophage plasticity and polarization: In vivo veritas: J Clin Invest, 2012; 122(3); 787-95
19. Liu YC, Zou XB, Chai YF, Macrophage polarization in inflammatory diseases: Int J Biol Sci, 2014; 10(5); 520-29
20. Shapouri-Moghaddam A, Mohammadian S, Vazini H, Macrophage plasticity, polarization, and function in health and disease: J Cell Physiol, 2018; 233(9); 6425-40
21. Yunna C, Mengru H, Lei W, Macrophage M1/M2 polarization: Eur J Pharmacol, 2020; 877; 173090
22. Wang LX, Zhang SX, Wu HJ, M2b macrophage polarization and its roles in diseases: J Leukoc Biol, 2019; 106(2); 345-58
23. Wu H, Yin Y, Hu X, Effects of environmental pH on macrophage polarization and osteoimmunomodulation: ACS Biomater Sci Eng, 2019; 5(10); 5548-57
24. McWhorter FY, Wang T, Nguyen P, Modulation of macrophage phenotype by cell shape: Proc Natl Acad Sci USA, 2013; 110(43); 17253-58
25. Wosik J, Suarez-Villagran M, Miller JH, Macrophage phenotype bioengineered by magnetic, genetic, or pharmacologic interference: Immunol Res, 2019; 67(1); 1-11
26. Li K, Lv L, Shao D, Engineering nanopatterned structures to orchestrate macrophage phenotype by cell shape: J Funct Biomater, 2022; 13(1); 31
27. Liu B, Zhang M, Zhao J, Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis: Exp Ther Med, 2018; 16(6); 5009-14
28. Xu M, Ji Y, Immunoregulation of synovial macrophages for the treatment of osteoarthritis: Open Life Sci, 2023; 18(1); 20220567
29. Zhang H, Cai D, Bai X, Macrophages regulate the progression of osteoarthritis: Osteoarthritis Cartilage, 2020; 28(5); 555-61
30. Zhu X, Lee CW, Xu H, Phenotypic alteration of macrophages during osteoarthritis: A systematic review: Arthritis Res Ther, 2021; 23(1); 110
31. Chen B, Hong H, Sun Y, Role of macrophage polarization in osteoarthritis (review): Exp Ther Med, 2022; 24(6); 757
32. Thomson A, Hilkens CMU, Synovial macrophages in osteoarthritis: The key to understanding pathogenesis?: Front Immunol, 2021; 12; 678757
Figures
 Figure 1. The structure of BGC and Cu-BGC scaffolds. The digital photographs of BGC (A) and Cu-BGC (E) scaffolds. The SEM images of BGC scaffolds (B–D) and Cu-BGC scaffolds (F–H).
Figure 1. The structure of BGC and Cu-BGC scaffolds. The digital photographs of BGC (A) and Cu-BGC (E) scaffolds. The SEM images of BGC scaffolds (B–D) and Cu-BGC scaffolds (F–H). Figure 2. The proliferation of macrophages. The proliferation of macrophages cultured with different concentrations of Cu-BGC (A) and Cu2+ ions (B) enhanced within certain concentrations range (n=6, * p<0.05, ** p<0.01, *** p<0.001, respectively).
Figure 2. The proliferation of macrophages. The proliferation of macrophages cultured with different concentrations of Cu-BGC (A) and Cu2+ ions (B) enhanced within certain concentrations range (n=6, * p<0.05, ** p<0.01, *** p<0.001, respectively). Figure 3. The expression of cytokines and surface markers in macrophages incubated with BGC and Cu-BGC. The expression of proinflammatory cytokines, IL-1β (A), IL-6 (B) and anti-inflammatory cytokine, IL-4 (C) of macrophages at day 3. The expression of M1 phenotype surface markers, CD86 (D), CCR7 (E), and M2 phenotype surface marker, Arg-1 (F) of macrophages at day 3. The relative gene amount of the CTR group was set as 1. * p<0.05, ** p<0.01, *** p<0.001, n=3.
Figure 3. The expression of cytokines and surface markers in macrophages incubated with BGC and Cu-BGC. The expression of proinflammatory cytokines, IL-1β (A), IL-6 (B) and anti-inflammatory cytokine, IL-4 (C) of macrophages at day 3. The expression of M1 phenotype surface markers, CD86 (D), CCR7 (E), and M2 phenotype surface marker, Arg-1 (F) of macrophages at day 3. The relative gene amount of the CTR group was set as 1. * p<0.05, ** p<0.01, *** p<0.001, n=3. Figure 4. The effect of Cu2+ ions on the expression of cytokines and surface markers in macrophages. The expression of proinflammatory cytokines, IL-1β (A), IL-6 (B), and anti-inflammatory cytokine, IL-4 (C) of macrophages cultured with different concentrations of Cu2+ ions at day 3. The expression of M1 phenotype surface markers, CD86 (D), CCR7 (E), and M2 phenotype surface marker, Arg-1 (F) of macrophages cultured with different concentration of Cu2+ ions at day 3. The relative gene amount of CTR group was set as 1. * p<0.05, ** p<0.01, *** p<0.001, n=3.
Figure 4. The effect of Cu2+ ions on the expression of cytokines and surface markers in macrophages. The expression of proinflammatory cytokines, IL-1β (A), IL-6 (B), and anti-inflammatory cytokine, IL-4 (C) of macrophages cultured with different concentrations of Cu2+ ions at day 3. The expression of M1 phenotype surface markers, CD86 (D), CCR7 (E), and M2 phenotype surface marker, Arg-1 (F) of macrophages cultured with different concentration of Cu2+ ions at day 3. The relative gene amount of CTR group was set as 1. * p<0.05, ** p<0.01, *** p<0.001, n=3. Figure 5. The morphology of macrophages incubated with BG and Cu-BG. The morphology of macrophages was examined by CLSM, including FITC (A, D), DAPI (B, E), and Merge (C, F). (scale bar=100 μm).
Figure 5. The morphology of macrophages incubated with BG and Cu-BG. The morphology of macrophages was examined by CLSM, including FITC (A, D), DAPI (B, E), and Merge (C, F). (scale bar=100 μm). Figure 6. The morphology of macrophages incubated with different concentration of Cu2+ ions. The CLSM images characterized the morphology of macrophages, including FITC (A), DAPI (B) and Merge (C). (scale bar=100 μm)
Figure 6. The morphology of macrophages incubated with different concentration of Cu2+ ions. The CLSM images characterized the morphology of macrophages, including FITC (A), DAPI (B) and Merge (C). (scale bar=100 μm) Figure 7. The degradation metabolism of chondrocytes. The expression of MMP3 (A), MMP13 (B) and Adamts5 (C) were co-cultured with 10 ng/ml IL-1β and Cu-BGC for 1 day. The expression of MMP3 (D), MMP13 (E) and Adamts5 (F) were co-cultured with 10 ng/ml IL-1β and Cu2+ ions for 1 day. The relative gene amount of CTR group was set as 1. * p<0.05, ** p<0.01, *** p<0.001, n=3.
Figure 7. The degradation metabolism of chondrocytes. The expression of MMP3 (A), MMP13 (B) and Adamts5 (C) were co-cultured with 10 ng/ml IL-1β and Cu-BGC for 1 day. The expression of MMP3 (D), MMP13 (E) and Adamts5 (F) were co-cultured with 10 ng/ml IL-1β and Cu2+ ions for 1 day. The relative gene amount of CTR group was set as 1. * p<0.05, ** p<0.01, *** p<0.001, n=3. Tables
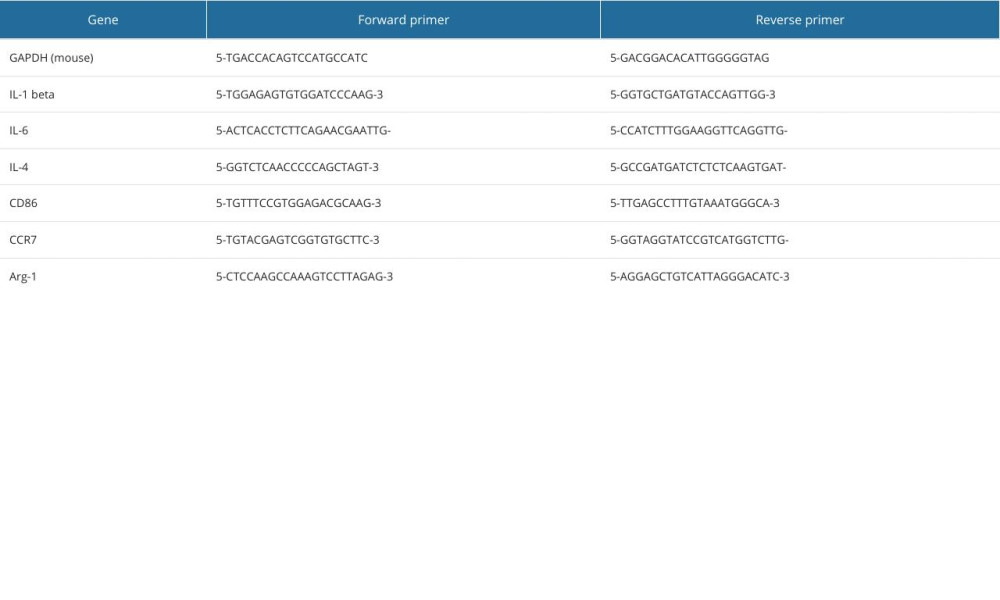 Table 1. The primer sequences used for RT-qPCR analysis.
Table 1. The primer sequences used for RT-qPCR analysis. Table 2. The RNA concentration of macrophages for RT-qPCR analysis by BGC and Cu-BGC.
Table 2. The RNA concentration of macrophages for RT-qPCR analysis by BGC and Cu-BGC. Table 3. The RNA concentration of macrophages for RT-qPCR analysis by Cu ions.
Table 3. The RNA concentration of macrophages for RT-qPCR analysis by Cu ions.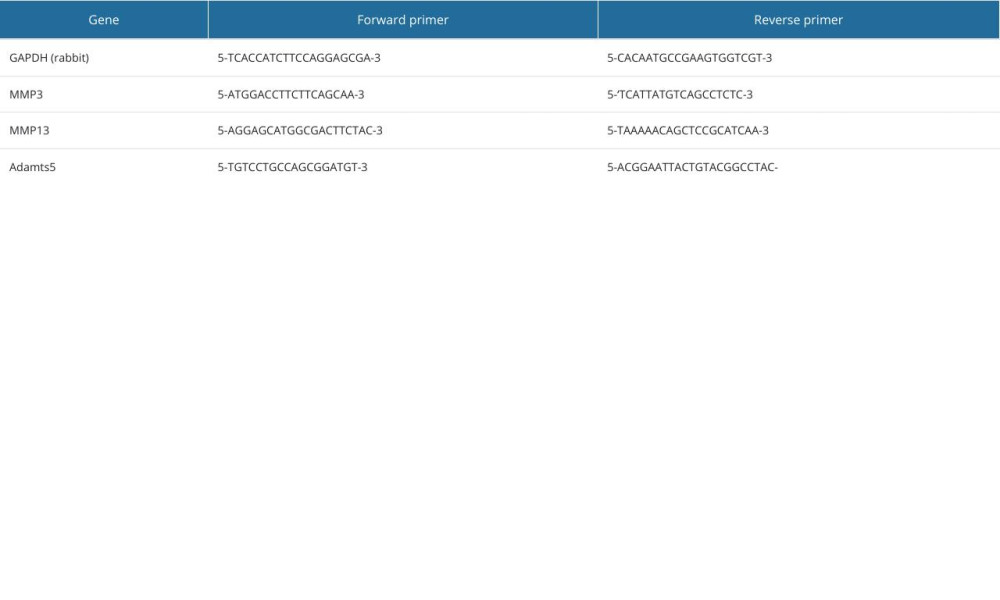 Table 4. The primer sequences used for RT-qPCR analysis.
Table 4. The primer sequences used for RT-qPCR analysis. Table 1. The primer sequences used for RT-qPCR analysis.
Table 1. The primer sequences used for RT-qPCR analysis. Table 2. The RNA concentration of macrophages for RT-qPCR analysis by BGC and Cu-BGC.
Table 2. The RNA concentration of macrophages for RT-qPCR analysis by BGC and Cu-BGC. Table 3. The RNA concentration of macrophages for RT-qPCR analysis by Cu ions.
Table 3. The RNA concentration of macrophages for RT-qPCR analysis by Cu ions. Table 4. The primer sequences used for RT-qPCR analysis.
Table 4. The primer sequences used for RT-qPCR analysis. In Press
12 Mar 2024 : Database Analysis
Risk Factors of Age-Related Macular Degeneration in a Population-Based Study: Results from SHIP-TREND-1 (St...Med Sci Monit In Press; DOI: 10.12659/MSM.943140
12 Mar 2024 : Clinical Research
Preoperative Blood Transfusion Requirements for Hemorrhoidal Severe Anemia: A Retrospective Study of 128 Pa...Med Sci Monit In Press; DOI: 10.12659/MSM.943126
12 Mar 2024 : Clinical Research
Tissue Inhibitors of Metalloproteinase 1 (TIMP-1) and 3 (TIMP-3) as New Markers of Acute Kidney Injury Afte...Med Sci Monit In Press; DOI: 10.12659/MSM.943500
12 Mar 2024 : Review article
Optimizing Behçet Uveitis Management: A Review of Personalized Immunosuppressive StrategiesMed Sci Monit In Press; DOI: 10.12659/MSM.943240
Most Viewed Current Articles
17 Jan 2024 : Review article
Vaccination Guidelines for Pregnant Women: Addressing COVID-19 and the Omicron VariantDOI :10.12659/MSM.942799
Med Sci Monit 2024; 30:e942799
14 Dec 2022 : Clinical Research
Prevalence and Variability of Allergen-Specific Immunoglobulin E in Patients with Elevated Tryptase LevelsDOI :10.12659/MSM.937990
Med Sci Monit 2022; 28:e937990
16 May 2023 : Clinical Research
Electrophysiological Testing for an Auditory Processing Disorder and Reading Performance in 54 School Stude...DOI :10.12659/MSM.940387
Med Sci Monit 2023; 29:e940387
01 Jan 2022 : Editorial
Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Pa...DOI :10.12659/MSM.935952
Med Sci Monit 2022; 28:e935952








